Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
H2 At The Tip Of STM Boosts Resolution
STM resolution of complex organic molecules can be greatly enhanced by modifying the tip
by Mitch Jacoby
September 6, 2010
| A version of this story appeared in
Volume 88, Issue 36

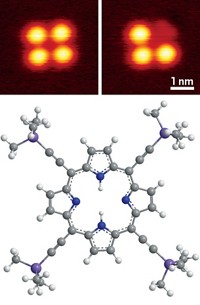
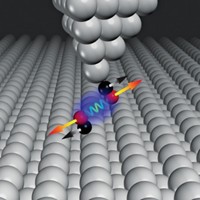
If you want to enhance the resolution of your scanning tunneling microscope, stick a hydrogen molecule on the tip. A team led by Ruslan Temirov of the Jülich Research Center, in Germany, reported in 2008 that STM resolution of complex organic molecules can be greatly enhanced by modifying the tip with hydrogen. But until now, the researchers didn’t understand why the trick works or what exactly the STM measures in that experiment. By repeating the experiments with deuterium and using quantum-mechanical calculations to analyze perylenetetracarboxylic anhydride supported on gold, the team obtained the answer (Phys. Rev. Lett. 2010, 105, 086103). As a result of its electronic structure and small size, a single molecule of deuterium or hydrogen confined between the probe tip and the organic molecule serves double duty, Temirov and coworkers say. They explain that D2 or H2 functions as a nanoscale force sensor because of short-range electronic repulsion between its electrons and those in the surface; it also acts as a transducer that converts this repulsive force into variations of the tunneling conductance.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter