Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Revealing How Plants Breathe
Structural Biology: An ion channel that controls opening, closing of pores in leaves has a rare protein fold
by Sarah Everts
November 1, 2010
| A version of this story appeared in
Volume 88, Issue 44

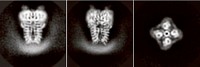
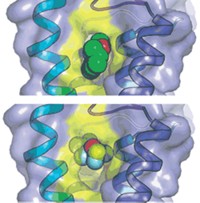
Just as plants take in the CO2 required for photosynthesis, they also release gas on a daily basis. Researchers have long wondered how pores in the leaf epidermis control this gas exchange. Now, a team of researchers at Columbia University has provided a first glimpse of an ion channel, called SLAC1, that helps open and close those pores (Nature 2010, 467, 1074). Its X-ray structure, which boasts a never-before-seen protein fold, may one day be useful for breeding or engineering plants that can better survive in arid environments.
“Every single carbon molecule in our bodies, our food, or our furniture has at one time gone through a plant’s pores—so has a significant portion of the water in our atmosphere,” comments Jaakko Kangasjärvi, a plant physiologist at the University of Helsinki, in Finland, whose group initially identified SLAC1. This ion channel controls passage through those pores, as well as plants’ response to drought conditions, changing CO2 levels, or even pathogen attacks, Kangasjärvi adds. “This structure will really help the plant biology community,” he says.
SLAC1 is buried in the plant cell membrane, making it hard to crystallize. Instead, researchers led by Wayne A. Hendrickson, a biochemist at Columbia University, crystallized the bacterial equivalent—a so-called homolog—of the plant protein. To show that the structure of the bacterial protein provides legitimate insight into the function of the plant protein, Hendrickson’s team did an extensive battery of biochemical experiments, including mutating essential residues and doing electrophysiology recordings of both the plant and bacteria protein reconstituted into frog egg cells, Kangasjärvi comments.
The X-ray crystal structure reveals that SLAC1 folds into a novel conformation that Hendrickson describes as “helical hairpins in a quasi-five-fold symmetry.” The protein sits in the membrane in groups of three. Each member of the threesome possesses a channel through which negatively charged ions such as chloride and nitrate slip, except when a phenylalanine side chain acts as a stopper. Opening of the channel is likely activated by phosphorylation, which causes conformational changes that “kick the phenylalanine out of the way,” Hendrickson says. When the channels are open, the efflux of negatively charged ions closes the pores in the plant epidermis.
The new structure narrows down which residues may be involved in the phosphorylation that opens the channel in plants, Kangasjärvi says. Pinpointing the precise phosphorylation sites that regulate the pore is just one of many questions that the plant biology community will use the new structure to answer, he adds.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter