Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Solar-Powered Electrochemistry
Organic oxidation reactions don’t need complicated setups or electricity from greenhouse-gas-emitting sources
by Michael Torrice
January 3, 2011
| A version of this story appeared in
Volume 89, Issue 1
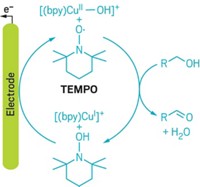
To make electrochemistry greener, Kevin D. Moeller and his group at Washington University in St. Louis have demonstrated that they can power their reactions with solar energy. Electrochemistry is inherently green because it allows chemists to limit chemical waste by avoiding oxidizing reagents or by recycling catalytic reagents such as transition-metal complexes. But many organic chemists think that electrochemistry requires expensive equipment and avoid it, Moeller said. His lab wanted to show that electrochemistry doesn’t need complicated setups or electricity generated from greenhouse-gas-emitting sources. The researchers bought small photovoltaic cells used to power toy cars and boats for less than $20 apiece. To run reactions, they connected the cells to the positive and negative electrodes in their flasks and then placed the setup in the light. Moeller and his team reproduced the yields of electrochemical reactions they ran previously with a conventional power supply, even difficult amide oxidations, he noted. So far, the reactions have involved direct oxidation of electron-rich compounds. Moeller’s next goal is to use the solar cells to recycle transition-metal reagents.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter