Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Trinitramide Debuts
In a first for the chemistry of nitrogen, researchers have detected the high-energy compound N(NO2)3
by Carmen Drahl
January 10, 2011
| A version of this story appeared in
Volume 89, Issue 2
In a first for the chemistry of nitrogen, researchers have detected the high-energy compound trinitramide, N(NO2)3, which until now has only been studied in theoretical contexts (Angew. Chem. Int. Ed., DOI: 10.1002/anie.201007047). Martin Rahm, Tore Brinck, and colleagues at the Royal Institute of Technology, in Stockholm, carefully nitrated ammonium dinitramide or potassium dinitramide at very low temperatures and detected trinitramide in both reactions by infrared and NMR spectroscopy. They also performed calculations to evaluate how trinitramide might decompose and to estimate some of trinitramide’s properties, such as density and heat of formation. The researchers suggest that trinitramide might be useful as a component of rocket propellants. Thomas M. Klapötke, an expert on nitrogen-rich compounds at Ludwig Maximilian University, in Munich, praised the discovery but says he isn’t sure that trinitramide will replace established propellant ingredients. Easily storable components are needed most, and it’s not clear that trinitramide will be stable above cryogenic temperatures, Klapötke says.


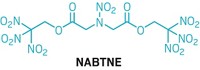
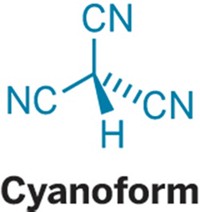
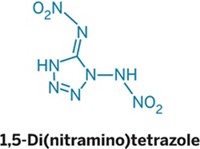

Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter