Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Kicking The Coffee-Ring Habit
Fluid Mechanics: Shift in particle shape suppresses vexing effect
by Bethany Halford
August 22, 2011
| A version of this story appeared in
Volume 89, Issue 34

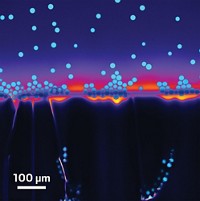
A change in particle shape, physicists have discovered, offers a simple way to circumvent the infamous coffee-ring effect (Nature, DOI: 10.1038/nature10344). The phenomenon is characterized by suspended particles accumulating at the edge of a drop of evaporating liquid. It gives coffee stains their distinctive dark edges and complicates the formulation of paints, coatings, and inks.
The problem occurs because most suspended particles are spherical, explains Peter J. Yunker, the graduate student who led the research effort, along with University of Pennsylvania colleagues Tim Still, Matthew A. Lohr, and Arjun G. Yodh. When a drop of liquid evaporates, fluid flows from the center of the droplet outward, taking the suspended spherical particles with it. Yunker and colleagues found that the coffee-ring effect did not occur with elliptical particles.
“Because of their elongated shape, ellipsoids deform the air-water interface,” Yunker explains. “To minimize the total deformation, ellipsoids pack together in clumps on the surface.” These clumps “are difficult to compress or push along the surface, so they prevent ellipsoids from reaching the edge” and result in a uniform deposition of particles.
But paint makers need not shift all their particle shapes from spherical to elliptical. Yunker and colleagues also found that under the right conditions, adding a small amount of elliptical particles to a suspension of spherical ones will also cancel the coffee-ring effect.
The results “may be of great practical importance,” notes Jan Vermant, a chemical engineering professor at Belgium’s Katholieke Universiteit Leuven, in a commentary about the work. He says Yunker’s discovery may also be relevant to things as “mundane as the food we eat or the consumer products we use.” Any item that comes as a cream or emulsion, Vermant says, is subject to the flow of thin films, which resembles the flow when a drop of liquid evaporates and causes the rupture of bubbles in foams or drops in emulsions. Yunker’s work may provide a way to better stabilize such products, he adds.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter