Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Quantum Chemical Calculations
October 31, 2011
| A version of this story appeared in
Volume 89, Issue 44
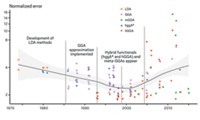
Although I agree fully with Vernon Box that caution should be exercised in using quantum chemical (QC) calculations, some of his comments need further clarification and expansion (C&EN, Sept. 26, page 2). For the past 49 years I have been actively involved in the development and application of a program for doing molecular orbital (MO) calculations, but within the past 20 years or so, I have noted that the evolution of density functional theory (DFT) and ab initio methods has virtually inundated the entire field of computational chemistry. Undoubtedly, DFT has come a long way in facilitating QC calculations. But since the software has become available to anyone, many problems in the applications and interpretations of these methods have been created.
Box makes a good point regarding the distinction between “long” versus “normal” bonds, but the amount of electron density between two potentially bonded atoms is a measure of only covalent bonding. However, if the bonding is primarily electrostatic, charge transfer, or polarizing in character, the bulk of the electron density is displaced toward the more negatively charged atom, or the one having the stronger polarizing strength. Thus a novice conducting QC calculations might easily misinterpret the significance of the electron density as it relates to bonding per se.
What bothers me even more are the claims among computational chemists, on the one hand, that computed energies in the range of ≤1 kcal/mol are regarded to be physically significant and having meaningful interpretation while, on the other hand, most computed electronic spectral transitions having energies hundreds of kcal/mol are computed via DFT with far less than 90% accuracy.
Unfortunately, there seems to be a distinct belief among most theoreticians that DFT and ab initio methods possess a quality like a “magic wand,” ensuring the most accurate results. Nonetheless, there is substantial evidence in the literature clearly showing that this is not the case. That very high precision is attainable does not ensure high accuracy. A long time ago, Dick Fenske (famous for the development of the Fenske-Hall MO method) personally told me that those who start to believe that their calculations are the most accurate must take a step back and eat some humble pie.
By Edward Boudreaux
Thornton, Colo


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter