Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Opioid Receptor Gang Of Four
Scientists obtain structures of the d-opioid and nociception receptors, rounding out analyses of the four opioid receptor subtypes
by Stu Borman
May 21, 2012
| A version of this story appeared in
Volume 90, Issue 21
\
\
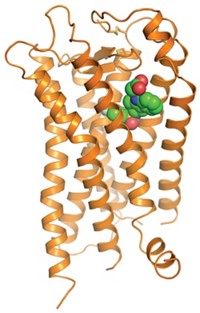
At top, a space-filling structure of the δ-opioid receptor bound to an antagonist (center), and at bottom a ribbon structure of the nociceptin receptor bound to an antagonist (spheres). Disks represent membrane surfaces.
Researchers have completed crystal structure analyses of the last of the four types of opioid receptors. These receptors are targets for antidepressants and pain and reward agents such as morphine. The structures were previously unavailable because opioid receptors, which are membrane proteins, would not form crystals. They were obtained through a technique that conjugates an opioid receptor to a crystallizable protein. This strategy led to antagonist-bound structures reported in March for the µ-opioid receptor by Brian K. Kobilka and Sébastien Granier of Stanford University and coworkers and the κ-opioid receptor by a group led by Raymond C. Stevens of Scripps Research Institute (C&EN, March 26, page 11). Now, the Stanford group has solved the δ-opioid receptor structure, and the Scripps team has reported the nociceptin (orphanin FQ) receptor structure, both bound to antagonists, rounding out the gang of four opioid receptor subtypes (Nature, DOI: 10.1038/nature11111 and 10.1038/nature11085). The crystal structures help elucidate how drugs bind the opioid receptors and could lead to optimization of the growing list of opioid therapeutics.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter