Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Uranium Complex Fuses CO To Make Furanones
Uranium triamidoamine complex stitches together CO molecules, suggesting CO polymerization might yet be possible
by Elizabeth K. Wilson
June 11, 2012
| A version of this story appeared in
Volume 90, Issue 24
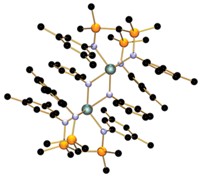
A simple uranium complex not only performs the challenging but potentially industrially valuable task of fusing together two CO molecules, but the complex is readily recycled after the reaction. Chemists have had difficulty finding an efficient way to manipulate the muscly C≤O bond and coax the molecule to form oligomers, such as the dimer ethyne diolate, –O–C≤C–O–. And even when metal complexes have been able to do the job, they have not been recyclable. Now, Stephen T. Liddle and colleagues at the University of Nottingham, in England, report a relatively simple process involving a triamidoamine uranium(III) complex that illustrates the potential for synthesizing functionalized CO homologs (Proc. Natl. Acad. Sci. USA, DOI: 10.1073/pnas.1203417109). Under mild conditions the uranium complex forms a species containing the O–C≤C–O building block. This molecule is then treated with an organosilyl halide to form a bis(organosiloxy)acetylene, which can be converted to a furanone (shown) in a ring-closing step. In addition, triamidoamine uranium(IV) iodide forms during the reaction, making it possible to create a cycle to restore the uranium(III) complex. “The simplicity of this system suggests that catalytic CO functionalization may soon be within reach,” Liddle and coworkers write.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter