Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Clearer Picture Of A Proteasome
Structural Biology: Arrangement of proteasome’s components provides clues to function
by Celia Henry Arnaud
January 16, 2012
| A version of this story appeared in
Volume 90, Issue 3

A new structural analysis of the yeast proteasome and one of its components promises to advance scientists’ understanding of this macromolecular machine, which breaks down proteins that are no longer needed. The proteasome is a key regulator of the cell cycle and a target for anticancer drugs.
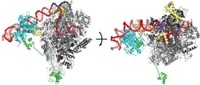
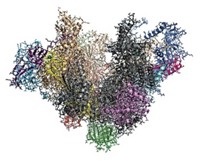
Larger than 1.5 million daltons, the proteasome consists of a barrel-shaped core, inside which proteolysis occurs, capped at one or both ends by structures known as regulatory particles, each of which is further divided into a lid and base.
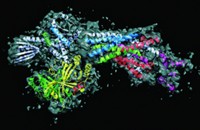
Biochemist Andreas Martin and coworkers at the University of California, Berkeley, report electron microscopy structures of the complete proteasome and an isolated lid at 9- and 15-Å resolution, respectively (Nature, DOI: 10.1038/nature10774).
The structures reveal that the lid sits on the side of the regulatory particle. In this orientation, it can interact extensively with both the regulatory particle’s base and the proteasome’s proteolytic core.
“The lack of a detailed structural model for the regulatory particle has held back research into the mechanism of how the proteasome operates,” says Raymond J. Deshaies, a biology professor at California Institute of Technology, who was not involved in the research.
The researchers found that ubiquitin receptors—which detect peptides that tag proteins for disposal—are located at the top of the complex, near an enzyme that removes the tags as the proteins are drawn into the core. The approximately 70–80 Å between the receptors and enzyme may explain why tags need at least four ubiquitin units, Martin says. Another group, led by Wolfgang Baumeister of the Max Planck Institute for Biochemistry, in Martinsried, Germany, has also carried out a structural study showing that the receptors are located at the top of the complex (Proc. Natl. Acad. Sci. USA, DOI: 10.1073/pnas.1119394109).
The conformation of the lid subunits in the complete complex is significantly different from their conformation in the isolated lid. These rearrangements could explain how the lid regulates the deubiquitinating enzyme, Martin says.
Particularly surprising in the structure is the spiral-staircase arrangement of six subunits in the base that unfold the protein substrate. These subunits, designated Rpt1–6, are all different, and the researchers were able to discern the position of each one. Martin says he and his coworkers expected a random organization of these subunits, but in every particle, Rpt3 was at the top of the staircase and Rpt2 was at the bottom. “We don’t know yet what it means mechanistically,” he says.
The structures could pave the way to developing inhibitors that target the regulatory particle instead of the core, which is currently a more common drug target. “It may be possible to inhibit degradation of certain substrates but not others,” Martin says.
“The regulatory particle has long resisted structural dissection,” Deshaies says. “One gets the feeling that this paper marks the beginning of the end, and it is now only a matter of time before a high-resolution atomic model is in hand.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter