Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
New Phosphinidene Transfer Reagents
Synthesis of phosphanorbornadienes opens new route to phosphorus(I) intermediates
by Stephen K. Ritter
September 3, 2012
| A version of this story appeared in
Volume 90, Issue 36
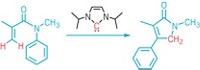
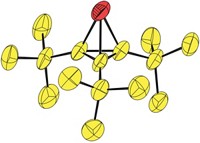
Chemists have discovered a simple procedure to make unprotected phosphanorbornadienes, a class of strained phosphorus compounds that has eluded isolation until now (J. Am. Chem. Soc., DOI: 10.1021/ja306902j). The achievement by Alexandra Velian and Christopher C. Cummins of Massachusetts Institute of Technology opens the way to using the phosphanorbornadiene group as a substituent in chemical synthesis. In prior efforts to synthesize phosphanorbornadienes, researchers assumed the molecules’ instability would require protecting the reactive lone pair of electrons on the unsaturated phosphorus atom by coordinating a metal or by forming a phosphine oxide. Velian and Cummins found that wasn’t necessary. They treated bulkily substituted phosphorus dichlorides (RPCl2) with the reducing agent magnesium anthracene to make unprotected dibenzophosphanorbornadienes (shown). These new compounds may serve as precursors to or transfer reagents for an array of unsaturated phosphorus intermediates known as phosphinidenes, which are phosphorus analogs of carbenes, Cummins says. “Mastering the chemistry of phosphinidenes offers the possibility of easily grafting phosphorus groups onto unsaturated polymeric materials and unsaturated drugs,” notes François Mathey, a phosphorus chemist at Nanyang Technological University, in Singapore.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter