Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Bacterial Protein Outwits Arsenate
Hydrogen bond drives preference for phosphate, with implications for arsenic-based life saga
by Carmen Drahl
October 8, 2012
| A version of this story appeared in
Volume 90, Issue 41
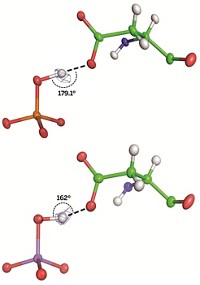
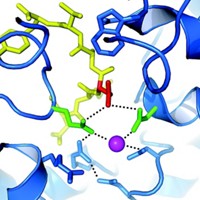
A distorted hydrogen bond shapes how a bacterial protein distinguishes essential phosphate from toxic arsenate, researchers report (Nature, DOI: 10.1038/nature11517). The work represents another nail in the coffin for the controversial claim that a microbe called GFAJ-1 can incorporate arsenate in its metabolites. Wondering how life evolved to deal with arsenate-rich environments, Weizmann Institute of Science postdoc Mikael Elias, Dan S. Tawfik, and colleagues turned to periplasmic phosphate-binding proteins, which regulate passage of phosphate anions through microbes’ inner membranes. They now find that versions of this protein from several bacteria, including GFAJ-1, are highly selective for phosphate. X-ray structures of the protein, from the bacterium Pseudomonas fluorescens, reveal that when arsenate binds, a hydrogen bond to an aspartic acid residue becomes distorted. Mutating the residue makes the protein less choosy for phosphate over arsenate, reinforcing the idea that the distortion drives the protein’s phosphate selectivity. Tawfik thinks that mechanism is also at play in the analogous protein from GFAJ-1, allowing it to survive in arsenate-rich environments. Scripps Research Institute biochemist Gerald F. Joyce says GFAJ-1 likely has multiple adaptations: “Now can we all stop talking about ‘arsenic-based life’?”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter