Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Peptide Scaffold Boosts MRI Contrast
Turning a gadolinium contrast agent into an amino acid and then forming a peptide improves image resolution
by Stephen K. Ritter
December 3, 2012
| A version of this story appeared in
Volume 90, Issue 49
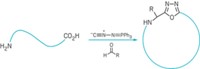
By adding an amino acid substituent to a macrocyclic ligand and incorporating it into peptides, Harvard Medical School scientists have found a way to tune the properties of gadolinium contrast agents to improve magnetic resonance imaging resolution at high magnetic fields (J. Am. Chem. Soc., DOI: 10.1021/ja309187m). The metal cation in an MRI contrast agent interacts with protons (hydrogen nuclei) in solvated water molecules, shortening the proton relaxation time after being pulsed by radiofrequency waves in a magnetic field. Better signal-to-noise ratios obtained at high magnetic fields lead to better image quality. But currently used contrast agents work well only at low fields. A team led by Peter Caravan figured out a way to stabilize a gadolinium macrocyclic complex so that the relaxation time remains optimally short at high magnetic fields. The researchers synthesized an amino acid analog by attaching alanine to the tetraaza macrocyclic ligand known as DOTA, and then they used solid-phase peptide synthesis to incorporate one or more of the alanine-modified complexes into linear or cyclic peptides. The rigid peptide scaffold inhibits the gadolinium complex’s rotation, enabling it to work better at high fields. The Harvard researchers showed that the modular metallopeptides outperform current clinical MRI contrast agents.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter