Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
New Detergents For Membrane Proteins
Amphiphiles improve stabilization and crystallization of membrane proteins for structural analysis
by Celia Henry Arnaud
March 18, 2013
| A version of this story appeared in
Volume 91, Issue 11
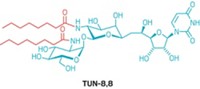
It’s a catch-22: Crystallizing membrane proteins requires the use of detergent molecules, but protein crystals grown with detergents are often of poor quality. To solve the dilemma, a multi-institutional team led by Qinghai Zhang of Scripps Research Institute California has improved upon a previously reported class of detergent molecules known as facial amphiphiles. The new amphiphiles stabilize membrane proteins from different families and form high-quality three-dimensional crystals (Proc. Natl. Acad. Sci. USA, DOI: 10.1073/pnas.1221442110). The molecules consist of sugar-containing chains attached to one face of the steroid cholate. The identity, number, and position of polar groups on the amphiphiles strongly affect the molecules’ ability to stabilize membrane proteins, the researchers note. The new amphiphiles associate tightly with membrane proteins to form small complexes that enhance the crystallizability of the proteins. The researchers crystallized various membrane proteins with the amphiphiles, including bacteriorhodopsin from Halobacterium salinarum, the transporter protein MsbA from Escherichia coli, the human channel protein connexin 26, and several cytochrome P450 enzymes.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter