Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
New Approach To Utilizing Lignin
ACS Meeting News: Wisconsin chemists develop a metal-free oxidation method for conversion of the biopolymer into useful aromatics
by Stephen K. Ritter
April 15, 2013
| A version of this story appeared in
Volume 91, Issue 15
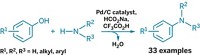
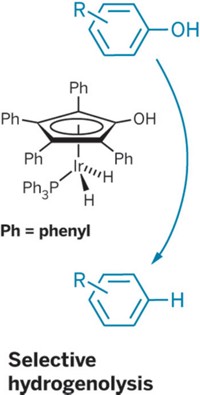
The irregular and complex structure of the biopolymer lignin, which surrounds cellulose and hemicellulose in plant cell walls, presents a challenge in trying to use the material as a biobased chemical feedstock. In an effort to improve lignin processing, Alireza Rahimi, Shannon S. Stahl, and coworkers at the University of Wisconsin, Madison, have come up with a stepwise approach to selectively oxidize lignin’s alcohol groups and then selectively cleave C–C and C–O bonds in the oxidized product to obtain valuable small aromatic compounds (J. Am. Chem. Soc., DOI: 10.1021/ja401793n). Rahimi described using an acetamido derivative of the nitroxyl radical compound TEMPO as an organocatalyst, along with molecular oxygen and mineral acid cocatalysts, to convert select alcohol groups to carbonyl groups in a lignin model compound. The oxidation set up the model compound for a subsequent peroxide-based oxidation that chops it into aromatic bits. The initial oxidation step also works well on lignin itself, Rahimi said. The next step for the Wisconsin team will be to try the cleavage step on oxidized lignin. They envision that the new process could serve as a platform for obtaining high yields of various aromatics from lignin by varying the reaction conditions of the stepwise oxidations.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter