Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Macrocycle Stars In Anion-Grabbing Action
Swiftly synthesized, star-shaped molecules pluck large anions from solution
by Bethany Halford
June 17, 2013
| A version of this story appeared in
Volume 91, Issue 24
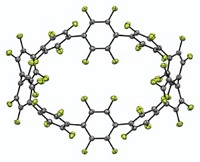
There’s a star on the rise in the world of supramolecular chemistry. Cyanostar, a new star-shaped macrocycle (shown), can pull weakly coordinating anions, which previously defied capture, out of solution (Nat. Chem. 2013, DOI: 10.1038/nchem.1668). The compound’s anion-sequestering abilities could find applications in environmental remediation of perchlorate and molecular sensing of biological phosphates. Although cyanostar is a neutral molecule, it possesses polarizable cyanostilbene-based C–H bonds that can coordinate large anions. It does so by forming sandwichlike structures, with the anion in the center capped by two cyanostars. The cyanostar, invented by Indiana University, Bloomington, chemists Amar H. Flood, Semin Lee, and Chun-Hsing Chen, is also easy to make. Its synthesis makes use of multiple Knoevenagel condensations, wherein benzylic nitriles react with aromatic aldehydes, followed by dehydration. The reaction proceeds with yields in excess of 80%, even on multigram scales, via a one-pot procedure. Such ease of synthesis is rare in the world of macrocycles, the Indiana chemists point out. The chemists also use cyanostar’s anion-binding powers to prepare a rotaxane with two cyanostars threaded on a dialkylphosphate moiety. Such structures are precursors to molecular machines.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter