Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Chemists Give Peptidomimetics An F
Kansas researchers demonstrate a new synthetic pathway for conveniently installing a fluoroalkene group in peptide-based drugs
by Stephen K. Ritter
August 12, 2013
| A version of this story appeared in
Volume 91, Issue 32
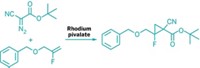
Organofluorine chemists have long appreciated that fluorinated alkene groups can serve as stand-ins for amide groups in modified natural peptides or synthetic peptides being explored as drugs. Fluorine provides distinct properties, such as decreased hydrogen-bonding abilities, which can improve the stability and bioactivity of the peptidomimetics. Ming-Hsiu Yang, Siddharth S. Matikonda, and Ryan A. Altman of the University of Kansas, Lawrence, have now come up with a convenient strategy for introducing fluoroalkene groups into peptide building blocks, steroids, and other bioactive molecules at a late stage in the synthesis (Org. Lett. 2013, DOI: 10.1021/ol401637n). The team went after the fluoroalkenes by altering the Shapiro reaction, which is widely used in natural product synthesis. This reaction typically involves condensing an N-sulfonyl hydrazide with a ketone, creating a vinyllithium intermediate, and then trapping the vinyl anion with H+ to obtain the alkene product. In the Altman group’s fluorination scheme, the researchers used N-fluorobenzenesulfonimide as a source of F+ to trap the fluoroalkene product. Given the number of ketone functional groups in natural products and pharmaceutically important building blocks, the researchers believe there’s a rich variety of substrates awaiting the new transformation.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter