Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Drug Discovery
Neuraminidase Inhibitors May Work When Tamiflu Doesn't
Drug Discovery: Agents block flu by a covalent mechanism, which could help prevent virus from becoming resistant
by Stu Borman
February 21, 2013
| A version of this story appeared in
Volume 91, Issue 8

The rise of influenza strains resistant to current flu treatments has drug developers looking for new leads. Hope could come from a new family of inhibitors that may effectively block the flu virus from spreading in the body and thwart its tendency to become drug-resistant.

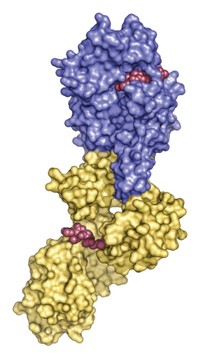
The new agents, devised by a University of British Columbia-based team, inhibit the flu virus enzyme neuraminidase. They halt the spread of flu viruses in cell culture and animal tests, including strains resistant to the commercial flu drug Tamiflu, which also inhibits neuraminidase. The compounds could therefore lead to a Tamiflu backup drug.
Flu afflicts millions worldwide each year, causing debilitating symptoms and hundreds of thousands of deaths. When the virus infects people, it enters airway cells and replicates. Progeny emerge as buds attached to sialic acids on cell surfaces. If neuraminidase cleaves these acids, the viruses are released to infect more cells. Inhibitors turn off this activity, blocking proliferation.
Four neuraminidase inhibitors are approved or in development for postinfection treatment. Tamiflu is the most popular, but flu can evolve into strains insensitive to it. Relenza is administered by oral inhalation, which has limited its use. Peramivir was withdrawn from a Phase III trial last year, and laninamivir is scheduled to enter Phase II, although both are approved in Asia.
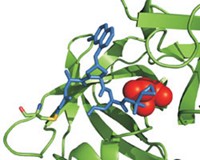
The new compounds emerged from efforts by Stephen G. Withers and coworkers to determine how neuraminidase works molecularly (Science, DOI: 10.1126/science.1232552). Their study shows that neuraminidase catalyzes sialic acid cleavage by a mechanism involving a covalent intermediate. They determined the structure of the intermediate and designed sialic acid analogs that bond covalently to the viral neuraminidase active site but release very slowly, thus disabling it, and do not inhibit human neuraminidase. The compounds may evade viral resistance more effectively than Tamiflu because their structures more closely resemble that of sialic acid. Also, covalent bonding permanently inactivates the neuraminidase active site; Tamiflu and the three other inhibitors bind noncovalently.
The Centre for Drug Research & Development, in Vancouver, is seeking private-sector partners and investors to help develop the new inhibitors commercially.
Drug-resistant viruses that cause pandemics “could be devastating for lack of any means to limit their spread or pathology,” comments chemical glycobiologist James C. Paulson of Scripps Research Institute, in California. The new inhibitors “should be developed as drugs for human testing that could be stockpiled or serve as alternatives.”
Relenza discoverer Mark von Itzstein of Griffith University, in Australia, says the new data “provide valuable insight into possible next-generation influenza drugs.”
Flu expert Larisa V. Gubareva of the Centers for Disease Control & Prevention, in Atlanta, is more cautious, saying that discovery of the covalent mechanism is noteworthy but the new inhibitors’ efficacy and advantages over existing inhibitors have yet to be fully evaluated.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter