Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Unsymmetrical Twist For NH-Acidic Amines
Framing an amine nitrogen with two different electron-withdrawing perfluoroalkyl groups yields a new weakly coordinating species
by Stephen K. Ritter
April 7, 2014
| A version of this story appeared in
Volume 92, Issue 14
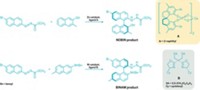
Amines or imides with two strongly electron-withdrawing groups, HNRR´, which are referred to as NH-acids, are popular molecules because they form very weakly coordinating anions. These species are essential components of some olefin polymerization catalysts and lithium-ion batteries. Researchers led by Julius F. Kögel and Jörg Sundermeyer at Philipps University, in Marburg, Germany, have devised a new member of this compound class (shown) that is unprecedented for bearing two different sterically demanding and strongly electron-withdrawing substituents: a pentafluorophenyl group and a perfluorinated tert-butyl group (Inorg. Chem. 2014, DOI: 10.1021/ic5001717). Among NH-acids, the most prominent example is bis(trifluoromethylsulfonyl)imide, HN(SO2CF3)2. In the past, chemists have made NH-acids with one SO2CF3 group paired with a C6F5 group or with a pair of pentafluorophenyl groups, but not with two different perfluorocarbon groups. The Marburg team prepared the new compound in three steps starting from pentafluoroaniline and hexafluoroacetone and studied the thermal, spectroscopic, and structural properties of its alkaline and alkaline-earth salts. The researchers suggest the new NH-acid will be useful in preparing ionic liquids, as a component of lithium-ion battery electrolytes, and as a weakly coordinating ligand in metal complexes.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter