Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Sulfate Click Chemistry
Researchers use SO2F groups as new links in simple selective reactions for forming carbon-heteroatom bonds
by Stephen K. Ritter
August 25, 2014
| A version of this story appeared in
Volume 92, Issue 34
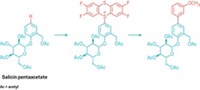
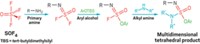
K. Barry Sharpless and his coworkers at Scripps Research Institute, in La Jolla, Calif., introduced the concept of click chemistry in the 1990s. Drawing largely on known reactions, the simple strategy involves using modular building blocks for the green synthesis of new compounds containing carbon-heteroatom linkages. Model reactions are copper-catalyzed azide-alkyne cycloadditions, which are useful in drug discovery, chemical biology research, and materials science. Sharpless and coworkers have now extended click chemistry by using SO2F functional groups prepared from sulfuryl fluoride (SO2F2) to make carbon-sulfate links, for example, in the synthesis of diarylsulfates (Angew. Chem. Int. Ed. 2014, DOI: 10.1002/anie.201309399). To demonstrate a potential commercial application, Sharpless, Valery V. Fokin, and colleagues clicked together SO2F2, bisphenol A, and various silyl chlorides to form polysulfates, a class of tough, high-clarity polymers related to polycarbonates that has not been widely studied because the macromolecules are difficult to make (Angew. Chem. Int. Ed. 2014, DOI: 10.1002/anie.201403758).


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter