Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Moving Toward Invisible Batteries
Wearable Electronics: A new solution-based, layer-by-layer assembly method forms ultrathin transparent electrode films
by Mitch Jacoby
September 28, 2015
| A version of this story appeared in
Volume 93, Issue 38
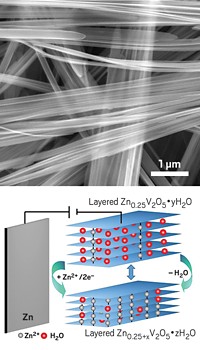
Wearable transparent electronics are moving from the concept stage to commercialization, thanks in part to the development of flexible, transparent displays. That transition may have just been quickened—and the range of design possibilities broadened—thanks to a study detailing a route to making transparent batteries (ACS Nano 2015, DOI: 10.1021/acsnano.5b03578). Typical procedures for making battery electrodes result in relatively bulky structures that aren’t see-through. Researchers led by Forrest S. Gittleson, Won-Hee Ryu, and André D. Taylor of Yale University have demonstrated that a solution-based electrostatically driven layer-by-layer assembly method can be used to fabricate ultrathin transparent lithium-ion battery electrodes. The team prepared transparent anode and cathode films by spraying solutions of single-walled carbon nanotubes and V2O5 nanowires, respectively, onto rotating substrates. They tested the anodes and cathodes separately in lithium-ion reference cells, verifying that the electrodes are electrochemically active and stable. They then coupled the electrodes to make a battery and showed that the test cell could retain its initial charge capacity for more than 100 charge cycles. The battery is still not completely transparent, however, because the electrode separator and current collector are not transparent. The Yale researchers plan to attack those issues next.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter