Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Microbiome
How your gut might modify your mind
The microbes that live in your body might be influencing your behavior. Researchers want to know what they’re saying to your brain and how
by Laura Howes
April 8, 2019
| A version of this story appeared in
Volume 97, Issue 14

Credit: Will Ludwig/C&EN
In brief
The idea that our guts may influence our mental health is not new, but in the past 15 years, it has gotten renewed attention from scientists. Amid the hype, researchers are beginning to solidify the connection between our gut microbiomes and our brains with studies mainly in mice. Questions remain about how gut-brain communication happens and whether it can be used therapeutically. But scientists say this field is still in its early days and are optimistic that we’ll find answers.
Anytime you touch a grimy handrail in the subway station or walk barefoot through the sand on a beach, you might be tempted to think that the skin we use to touch and feel is the largest interface with our surroundings. But you would be wrong; our guts are much bigger.
Curled up inside us, our intestines have a surface area of around 32 m2, or 344 ft2. Put another way, the intestines of an average adult occupy the same area as a small studio apartment in New York City. But instead of housing a stressed-out Manhattanite, a human’s gut hosts trillions of microbes. Our intestinal walls absorb and interact with all the molecules we ingest, but so do these microscopic chemists that live inside us. They take the nutrients in and then pump out a raft of new chemicals.
This community of bugs is diverse and relatively stable—an ecosystem of bacteria, viruses, and fungi. In exchange for raw materials and shelter, the microorganisms, collectively known as the microbiome, feed and protect their hosts.
But the influence of our microbial community doesn’t stop there. Studies have shown that our microbiome may play a role in mental health and neurological conditions such as autism, epilepsy, and depression by interacting with our nervous system and even releasing molecules that can perhaps make their way to the brain. More research and trials are needed to understand how the gut and the brain are linked, but researchers suggest that their findings might one day lead to treatments for neuropsychiatric disorders.
Possible pathways
The mechanisms connecting the gut and brain are still unclear, but here are some of the most popular ideas.
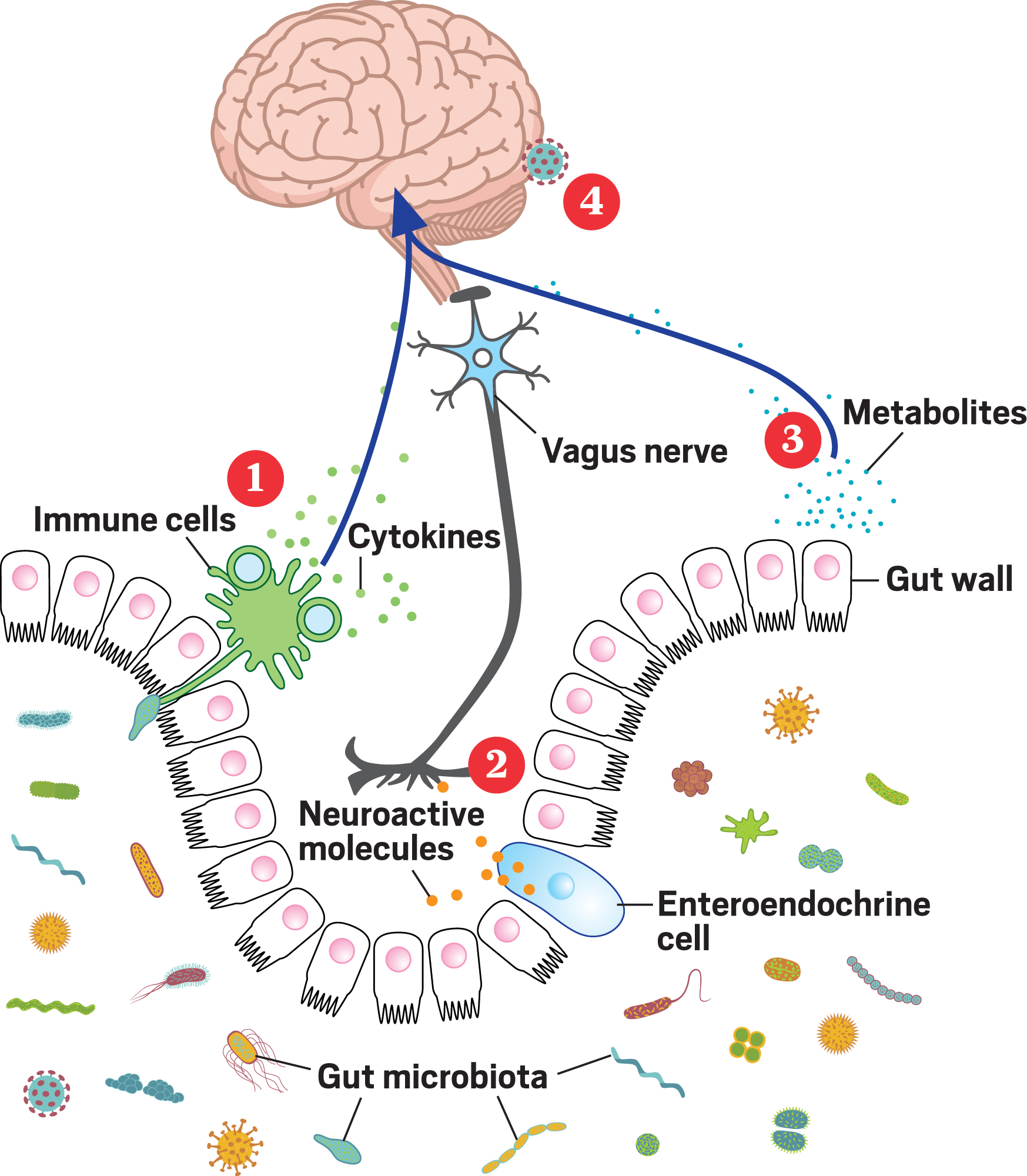
1. Microbes interact with immune cells in the gut, prompting the cells to make cytokines that circulate from the blood to the brain.
2. Microbes interact with gut cells called enteroendocrine cells that produce neuroactive molecules and peptides. These molecules interact with the vagus nerve, which sends signals to the brain.
3. Microbes in the gut produce neurotransmitters and metabolites like butyrate. These circulate to the brain, where some of them are small enough to cross the blood-brain barrier, and others alter cell activity at the barrier itself.
4. In 2018 researchers at the University of Alabama at Birmingham reported at a meeting that they had found gut bacteria in human brain tissue. The study has not yet been published, and skeptics abound, but it suggests that microbes might somehow be making their way into the brain.
Source: Adapted from Front. Integr. Neurosci. 2013, DOI: 10.3389/fnint.2013.00070.
Feeling it in your gut
Doctors have been wondering about the links between digestion and mental health since the 19th century. Inspired by famed scientist Louis Pasteur, who speculated in 1885 that animals lacking bacteria would die, European doctors began investigating the significance of microbes located in the digestive system. Perhaps, the doctors suggested, “toxins” produced by microbes in the gut were poisoning the minds of their patients. This area of study became hugely popular for a few decades before being discredited, and medical science moved on.
Until as recently as 2004, the suggestion that bacteria in the human gut could be a factor in mental health was regarded with suspicion. In that year, Nobuyuki Sudo’s group at Kyushu University, in Japan, reported that the bodies of so-called germ-free mice reacted strongly to stress compared with those of non-germ-free mice (J. Physiol. 2004, DOI: 10.1113/jphysiol.2004.063388). Germ-free mice are lab animals raised in an isolated environment so they have no microorganisms living inside or on them.
While the paper didn’t immediately gain much attention, since then, evidence has slowly been mounting that intestinal microbiota are linked to mood, behavior, and cognition. Among scientists, the report is now viewed as the start of a new field of research: the study of the gut-brain connection.
In 2005, John F. Cryan, a neurobiologist with expertise in how stress affects the brain, joined the faculty at University College Cork, which was already home to many researchers interested in stress and its role in the gut—specifically, how it is involved in irritable bowel syndrome.
By 2009, Cryan’s group published a study showing that when young rat pups were separated from their mothers, the stress early in life could lead to long-term changes in the animals’ microbiomes (Biol. Psychi. 2009, DOI: 10.1016/j.biopsych.2008.06.026). “It was just one of, like, 10 different measures we took to really prove that stress was a whole-body syndrome,” Cryan says. So to begin with, the finding didn’t particularly stand out in the crowd, but it did get Cryan thinking.
He began to search the literature for other links between stress and the gut microbiome and found the 2004 paper from the Japanese team. At that point, he says, the paper was still not very well known, but it helped him make some connections. His own team had shown that stress affects the microbiome in mice. The Japanese team had shown that the microbiome affects how mice deal with stress. Cryan and his team investigated the link further.
In 2011, they reported that when a probiotic bacterium known to influence the immune system, Lactobacillus rhamnosus JB-1, was given to a mouse, the animal’s stress behaviors decreased and its brain chemistry changed (Proc. Natl. Acad. Sci. U.S.A. 2011, DOI: 10.1073/pnas.1102999108). Mice with a severed vagus nerve did not get the same benefits when given L. rhamnosus JB-1, however. “That was a big kind of breakthrough moment for us in relation to this field,” Cryan says.

What happens in vagus
The vagus nerve is like a superhighway between the gut and the brain. One of the 12 major nerves that directly connect the body to the brain, its name comes from the Latin for “wandering,” because it links dozens of parts of the body to the medulla oblongata, a grape-sized bit of tissue in humans that’s located at the base of the brain. With all its connections, the vagus nerve gives us a sense of how our bodies are doing. “Sometimes you just feel good; sometimes we feel crappy. That is your vagus nerve telling you what’s going on,” Cryan explains.
95%:
Percentage of the human microbiome that lives in our gastrointestinal tract
~10 trillion:
Number of microbes living in our guts
0.2 kg:
Mass, for an average person, of all the microbes in our guts
80–90%:
Percentage of the vagus nerve that sends information from the body to the brain
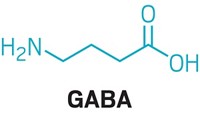
When Cryan’s lab fed L. rhamnosus JB-1 to laboratory mice in 2011, the animals produced less stress-induced corticosterone in their bodies and displayed fewer anxiety- and depression-related behaviors. Feeding the mice the bacteria also changed the amount of a particular type of protein receptor produced in different parts of the brain. This receptor binds to the neurotransmitter γ-aminobutyric acid (GABA).
The exact mechanism by which the gut microbiome interacts with the vagus nerve isn’t known. But, Cryan says, the fact that these changes didn’t happen in animals that had their vagus nerve cut is evidence that it is definitely involved in communicating between the gut microbiome and the brain.
Other researchers have also noticed the vagus nerve’s link between the gut and the brain. Around 2015, Mauro Costa-Mattioli and his colleagues at Baylor College of Medicine were investigating a link between a mother’s diet and the development of autism in her children when they inadvertently stumbled into gut microbiome research. “Obesity is a big issue here in the US,” Costa-Mattioli explains. “And there are epidemiological studies that actually showed that if the mom is obese, there is a higher possibility of the offspring developing autism.”
Costa-Mattioli’s team fed female mice a high-fat diet to make them obese before they got pregnant and then examined the behavior of their offspring after they were born. They found that mice from obese mothers displayed one of the symptoms of autism spectrum disorders—namely, problems with social interaction. Or at least they did initially. Once the animals were weaned, the control animals and the experimental mice were put in the same housing. Four weeks later, all the mice were interacting normally.
“Of course we scratched our brains for months trying to figure out what was going on,” Costa-Mattioli recalls. Eventually, the researchers realized that the maternal diet induced a shift in the offspring’s microbiome. But mice typically eat one another’s poop. So putting the two groups together in the same housing meant the mice ingested each other’s microbiota, and the asocial mice became social. Sequencing the DNA of the bugs in the guts of the animals before and after mixing the groups pointed to this explanation.
“To make a long story short, what we discovered is that the maternal diet eliminates a particular bacterium, Lactobacillus reuteri, in the offspring,” Costa-Mattioli explains. Reintroducing the bacterium, either by intervention from the scientists or by the mice eating poop, reverses the social deficit. The scientists also found that the bacterium sends signals from the gut to the brain via the vagus nerve, increasing production of the hormone oxytocin, which promotes social bonding (Cell 2016, DOI: 10.1016/j.cell.2016.06.001).
Bugs as drugs?
Of course, any discussion of how diet might influence the microbiome and thus alter mental health naturally leads to the idea of therapeutics. In 2013, Cryan coined the term psychobiotic to describe a medication containing live bacteria or their metabolic products that might confer a mental health benefit on humans.
In fact, many argue that psychobiotics have been around for a long time and that physicians have already been using them without even realizing it. Since the early 1900s, neurologists have known that putting children with epilepsy on a specific diet can reduce their seizures. This so-called ketogenic diet is low in carbohydrates and high in protein and fat. But scientists have never really understood how it works.
Elaine Hsiao, a microbiologist at the University of California, Los Angeles, suspected that the answer might lie in the gut microbiome. In 2018, she showed that two particular types of gut bacteria thrive in mice feasting on a ketogenic diet (Cell 2018, DOI: 10.1016/j.cell.2018.04.027). Hsiao and her team think these bugs might be providing their hosts with building blocks for neurotransmitters such as GABA. In the brain, GABA acts like a sort of brake for brain cells, reducing the activity of neurons and keeping networks properly balanced.
“This discovery has the potential to impact the many conditions that are associated with alterations in GABA and shown to be modified by the ketogenic diet,” Hsiao says. She lists epilepsy, Alzheimer’s disease, Parkinson’s disease, autism, anxiety, and schizophrenia as potential conditions to target. To test whether the ketogenic diet could benefit people with these other conditions and bring treatments to the market, Hsiao started a company, Bloom Science.
Across town at the California Institute of Technology, scientists have started up another firm, Axial Biotherapeutics, that hopes to develop psychobiotics too. In 2016, microbiologist Sarkis K. Mazmanian and colleagues pinpointed short-chain fatty acid signaling as mediating some of the gut’s effects on the brain (Cell 2016, DOI: 10.1016/j.cell.2016.11.018). Using mouse models of Parkinson’s disease, the researchers found that giving the mice microbes taken from the guts of people with Parkinson’s made the animals’ symptoms worse. The same happened if the mice were given short-chain fatty acid metabolites from the bugs.
From mice to humans
But not everyone is convinced that scientists should even be discussing treatments at this stage. Katarzyna B. Hooks, a computational biologist at the University of Bordeaux, believes that a lot of the claims linking the gut and the brain overreach. Although she sees the field as having promise, Hooks thinks it’s too soon to expect a magic bullet treatment.
Hooks recently coauthored a critique of the field (Behav. Brain Sci. 2018, DOI: 10.1017/S0140525X18002133). In the review article, Hooks, Bordeaux neuroscientist Jan-Pieter Konsman, and Maureen A. O’Malley, who studies the philosophy of microbiology at the University of Sydney, argue that links between the gut microbiome and the brain have been accepted without enough investigation and skepticism. “It would be fair to say that we are simply skeptical about the idea of psychobiotics,” Hooks says of herself and her coauthors.
“The relationship between gut microbial metabolism and mental health is a controversial topic in microbiome research,” says Jeroen Raes of KU Leuven. “Gut microbiome–brain communication has mostly been explored in animal models, with human research lagging behind.” And anyone paying attention to the field of brain science knows that observations in mice aren’t always reproduced in humans.
With the rise of DNA sequencing, however, researchers like Raes can now begin to focus on humans. In February, Raes’s group announced that, via sequencing, it had identified specific groups of microorganisms that positively or negatively correlate with mental health in humans (Nat. Microbiol. 2019, DOI: 10.1038/s41564-018-0337-x). Using samples from more than 1,000 participants in the Flemish Gut Flora Project, the authors found that two bacterial genera, Coprococcus and Dialister, were consistently depleted in individuals with depression.
Advertisement
While studying Coprococcus and Dialister, the team noticed these bacteria harbor genes coding for butyrate, a short-chain fatty acid that is known to have antidepressive properties. But as Bordeaux’s Konsman points out, Raes’s work does not demonstrate how butyrate-producing bacteria in the gut might influence depression. Just because those bacteria have genes coding for butyrate doesn’t mean the genes are being expressed and butyrate is being produced, he says. And even if it is produced, the fatty acid or its metabolites would need to cross both the gut barrier and the brain barrier to affect brain processes directly.
What Raes’s paper does show, Konsman and Hooks say, is a correlation between the population of the gut microbiome and depression. “It’s correlation,” which isn’t certainty, Cryan says, “but it’s a really good start.”
Cryan agrees with critics who argue that more human research is needed to firmly establish a gut-brain connection, but he is also firm in his belief that animal models still have a role to play. “We’re really at the beginning of figuring all of this out. And it’s easier to do that in mice where we control the diet and we control the repertoire of chemicals that are present,” he says. Studies become much more complex when you move to humans, Cryan adds, “and I think that’s where we’re going to see a lot of the hurdles, in interindividual differences.” Mouse populations in the lab can be rather homogeneous, but human populations are anything but.
It’s incredibly appealing to think that adjustments to diet or your gut microbiome could help treat neurological disease. That may be why the field has attracted so much public attention. But researchers urge caution. “The main thing is that we have to be careful of the hype,” Cryan says. Some companies, he says, will use studies such as the ones discussed in this article, without an understanding of the mechanisms connecting gut and brain and without clinical trials, to sell their probiotic supplements for treating diseases such as depression. “There’s a lot of snake oil” surrounding the field, he says. “We have to be really careful about that.”
For his part, Costa-Mattioli stresses that he is working on basic science rather than immediately thinking about drugs and therapeutics. For instance, he doesn’t think the parents of autistic children should buy over-the-counter probiotics, believing they will be a solution. But perhaps one day, he suggests, treatments might have a microbial component. “I always tell my students, ‘Don’t oversell the stories, but, yes, be enthusiastic when you can be because this is amazing.’ I could never imagine in my wildest dreams that a bacteria would be able to make animals more social.”
He says he always welcomes his critics to present him with an alternative scenario or explanation for his findings. “I think that what we need is more time,” he says. “And time will tell.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter