Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Education
Newscripts
Lego bricks for cutting-edge physics and for chemistry education
by Kerri Jansen
March 28, 2020
| A version of this story appeared in
Volume 98, Issue 12
An extremely cool experiment
Here at Newscripts, we’ve always thought Lego sets were cool. Now, we have peer-reviewed literature to back that up. In a paper published late last year, graduate student Josh Chawner and colleagues at Lancaster University report chilling a set of Lego bricks to near absolute zero. Although it started as merely a fun experiment, the venture yielded results that could actually be useful to physicists. It’s a frigid new frontier for the Danish brick sets (Sci. Rep. 2019, DOI: 10.1038/s41598-019-55616-7).
Chawner tells Newscripts that he and senior researcher Dmitry Zmeev hatched the idea for the experiment at a local pub. The two are part of Lancaster’s low-temperature physics group, which uses a custom-built dilution refrigerator to reach the extremely low temperatures needed to do the experiments. Starting when he was a teenager, Chawner has been creating elaborate animated videos starring Lego characters in a variety of adventures, such as escaping bootleggers in Prohibition-era New York. Chawner and Zmeev recognized their unique opportunity to create the next great Lego-adventure video, sending a Lego “cryonaut” into the refrigerator to become the world’s coldest minifigure.
“It was a bit controversial,” Chawner says. Colleagues worried that the plastic might leach heat after the refrigerator was chilled down, potentially interfering with other experiments. Fortunately, that wasn’t an issue, Chawner says. Researchers nestled the minifigure inside the machine, then lowered its temperature to around 4 millikelvins.

The researchers also decided to chill a stack of Lego bricks to test the plastic’s thermal conductivity. Good insulator materials are critical for supercool experiments and technologies like quantum computing. To their surprise, they found the stack of inexpensive bricks provided better thermal isolation than the pricier high-performance plastics typically used.
Any residual skepticism about the experiment seems to have evaporated once Chawner shared a draft of the paper with colleagues around his department.
“The next day I went into the lab and they all came in at once and were like, ‘This is brilliant! I knew we should have done this!’ ” Chawner recalls. He says he now plans to include a chapter on the experiment in his thesis.
A Lego chemistry set
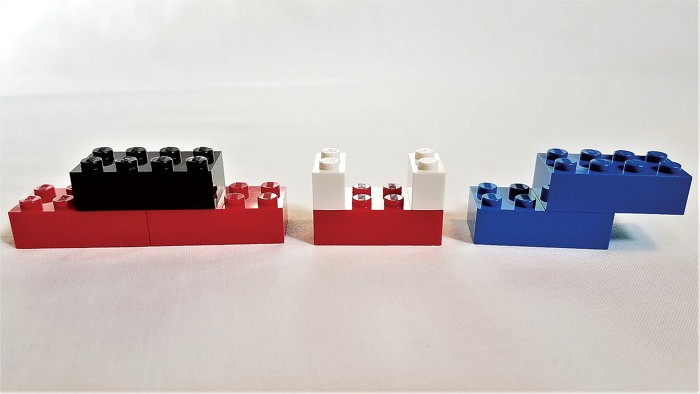
Lego bricks can be useful for science at room temperature, too. With coronavirus-related school closures keeping many kids at home, parents may want to consider pulling out Lego bricks and checking out atomic activities from the Massachusetts Institute of Technology’s Edgerton Center.
Kathleen M. Vandiver, an outreach and engagement director at MIT, developed the set and activities in the late 1990s when she was teaching sixth-grade science. Vandiver knew the value of hands-on activities for teaching abstract concepts and thought Lego bricks would be a perfect tool to teach that atoms are the building blocks of nature.
“I realized that the kids didn’t need to know all the bonding details in the beginning,” she says. “They just needed to know that atoms are everywhere, they’re natural, and also that they combine and stick together in predictable groupings.”

Vandiver used various colors of Lego bricks to represent various atoms, which kids could then assemble into simple molecules. When possible, Vandiver assigned Lego atoms the same colors that are used in more advanced molecular models—carbon is represented with a black brick, for example. Parents can use masking tape and markers to temporarily give bricks a new color or purchase specific items from an online retailer like BrickLink. Lego lessons include concepts like photosynthesis, air pollution, and chemical reactions in the ocean, and they are designed for kids 11 and up. Parents can download printable instruction mats and worksheets at edgerton.mit.edu/molecule-set.
And Vandiver notes another nice thing about Lego bricks, given the current situation: the plastic pieces are easily cleaned with soap and water.
Kerri Jansen wrote this week’s column. Please send comments and suggestions to newscripts@acs.org.
UPDATE:
This story was updated on March 3, 2023, to restore a missing paragraph in the story about a Lego chemistry set. The paragraph introducing Kathleen M. Vandiver had been omitted because of a production error.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter