Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Energy
Studying why fuel cells slow down
Computer simulations provide mechanistic details that could help researchers replace costly platinum in fuel cells
by Cici Zhang
June 26, 2018

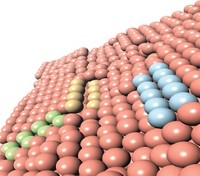
As fuel-cell cars running on hydrogen gas start to drive off car lots, researchers continue to find ways to lower the sticker price of these vehicles that emit just water vapor. One issue affecting their price is the use of expensive platinum metal as catalysts in the fuel cells that react hydrogen and oxygen to generate electricity. Replacing the platinum with less costly, nonprecious metals currently comes with a downside—a loss of efficiency in those electrochemical reactions. Now a team at California Institute of Technology has combined computer simulations and experiments to explain why the reactions run less efficiently. Understanding this mechanism, the researchers say, could help design fuel cells that work with less platinum or other metals without sacrificing efficiency.
Inside fuel cells, two chemical reactions generate the flow of electrons that can power a vehicle’s engine: hydrogen oxidation at the anode and oxygen reduction at the cathode. These reactions often run under acidic conditions, but to replace the state-of-the-art platinum catalysts with other metals, engineers have to raise the cell’s pH. At basic pHs, the hydrogen oxidation reaction is 100 times slower than at an acidic pH, even on platinum. Although researchers have proposed multiple hypotheses to explain these sluggish reaction rates, no one had nailed the mechanism.
Now, using quantum mechanics simulations, a team led by William Goddard has solved the long-standing mystery: They found that at a high pH, the catalyst’s surface is more negative than at a low pH, and that charge repels water molecules and makes the hydrogen atoms produced by splitting hydrogen gas bind more strongly to the surface. That stronger binding makes the atoms more stable and less willing to give up their electron and leave the surface as H+ ions to complete the hydrogen oxidation reaction. Essentially, the high pH creates a giant speed bump for the reaction. The researchers determined that their simulation data—the hydrogen binding energies at different pH levels—matched well with experimental measurements (J. Am. Chem. Soc. 2018, DOI:10.1021/jacs.8b04006).
Advertisement
Understanding this pH-dependent activity can guide design of cheaper fuel-cell catalysts that work in a basic medium, researchers who are not involved in the study say. For example, researchers could search for nonprecious metal catalysts that have more optimal hydrogen and water binding energies at a high pH, says Yushan Yan of the University of Delaware who studies electrocatalysis. The Caltech team also proposes modifying the catalyst’s surface properties by adding certain alloys or surface groups to improve efficiency.
The mechanism is also important for designing electrolyzers, devices that generate hydrogen from water. To make such hydrogen generation reactions run faster, Goddard says, researchers will want to increase water adsorption to the surface of a catalyst and decrease hydrogen binding energies. Electrochemist Juan Herranz at the Paul Scherrer Institute says that electrolyzers and fuel cells “are meant to play a crucial role in our near future, and the lessons taught by this paper will hopefully serve to make better-performing catalysts and to speed up the development of these clean technologies.“.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter