Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Hydrogen Power
Catalyst frees hydrogen from seawater
New solar-powered electrolysis system avoids briny bugbears like chlorine production
by Mark Peplow
March 30, 2018
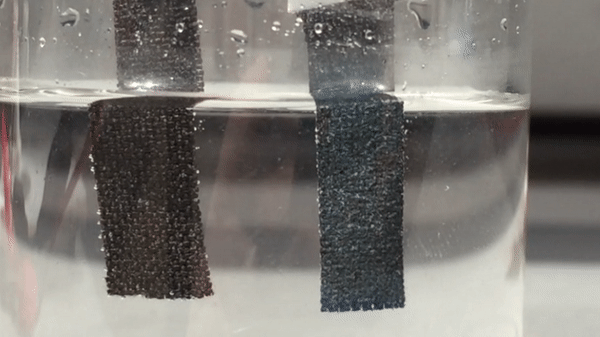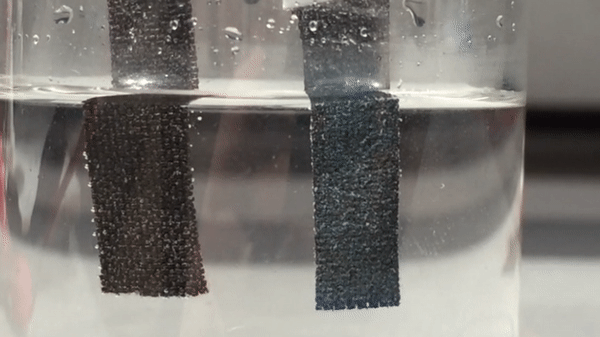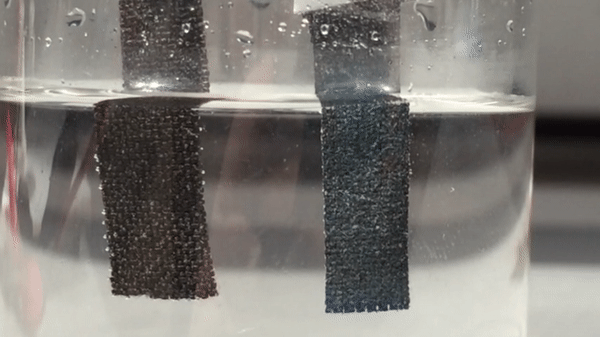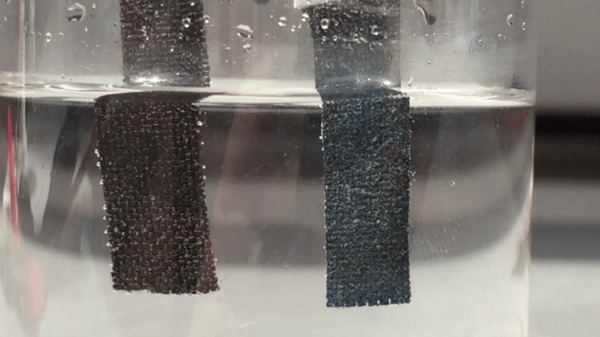
Harvesting hydrogen gas from water through electrolysis could lead to a renewable source of fuel. For a small island nation like Singapore, though, fresh water is a precious resource. So electrolysis researchers there have turned their attention to the sea. They have now developed a catalyst that helps to electrolyze seawater with record-breaking efficiency, generating oxygen and hydrogen that could eventually feed fuel cells (Adv. Mater. 2018, DOI: 10.1002/adma.201707261).
The system is powered by solar electricity, producing hydrogen from sunlight with an overall efficiency of 17.9%. “As far as we know, that’s the highest efficiency for seawater,” says Bin Liu of Nanyang Technological University, who was part of the research team.
The oceans contain a vast store of hydrogen atoms, but freeing them by electrolysis is a huge challenge. In an electrolyzer, the current used to split briny water usually turns chloride ions into unwanted chlorine gas, while other ions like calcium and magnesium form insoluble precipitates that clog vital catalysts on the electrodes. The electrolysis reactions can also cause pH changes that corrode electrodes.

Liu’s team had previously developed a nickel molybdenum sulfide catalyst that lowered the voltage needed to generate hydrogen gas at a cathode from seawater (Sci. Adv. 2015, DOI:10.1126/sciadv.1500259). Their new catalyst relies on earth-abundant elements to oxidize seawater at an anode, producing oxygen gas, protons, and electrons.
To make the anode, the researchers grew nanoneedles of basic cobalt carbonate on carbon fiber cloth. Then they dipped the cloth in 2-methylimidazole, which formed a thin layer of a cobalt-imidazole metal organic framework (MOF) on the outside of the needles. Adding sodium ferrocyanide transformed that layer into cobalt hexacyanoferrate, which inherited the porous nanostructure of the MOF and formed 20-nm-thick catalytic shells around the conducting nanoneedles.
With a commercial triple-junction solar cell to supply electricity, the team tested the system using local seawater, adding nothing more than a phosphate buffer to maintain a neutral pH. After 100 hours of continuous operation, the electrolyzer had made hydrogen and oxygen, but no chlorine at all. Moreover, its electrodes and catalysts were intact, and its output had fallen by just 10%. In contrast, an electrolyzer that used conventional catalysts of platinum and iridium oxide to split the local seawater lost activity much more rapidly, and also produced some chlorine.
“The fact that it is selective for oxygen evolution rather than chlorine evolution is very significant,” says Michael E. G. Lyons, an electrocatalysis researcher at Trinity College Dublin. “It’s a very tricky thing to do.”
Peter Strasser at the Technical University of Berlin, who has worked on seawater electrolysis, points out that the system has a very low current density. To make useful amounts of hydrogen, the system would need a much higher current density, which could trigger chlorine evolution or other unwanted side reactions. “Problems arise when you go to high current densities,” he says.
Advertisement
Liu says that initial tests at higher current densities have not produced any chlorine. But he acknowledges that the system’s performance could be improved. Using fresh water, for example, solar-powered electrolyzers have reached solar-to-hydrogen efficiencies of more than 30% (Nat. Commun. 2016, DOI: 10.1038/ncomms13237). Liu’s team is now working with researchers at the Dalian Institute of Chemical Physics to develop their system into a prototype device for generating hydrogen.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter