Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Hydrogen Power
Nanostructures help chemists generate hydrogen in microgravity
Textured catalyst prevents gas bubbles from clinging to surfaces
by Kerri Jansen
July 11, 2018
| A version of this story appeared in
Volume 96, Issue 29

Long-term space missions will require methods to produce fuels, such as hydrogen gas, on demand. Photoelectrochemical cells (PECs) can produce hydrogen using energy from sunlight, but the reduced gravity in space presents unique challenges to this chemistry.
An international team of researchers led by California Institute of Technology’s Hans-Joachim Lewerenz studied one of these low-gravity problems and demonstrated how a nanostructured catalyst surface could solve it (Nat. Commun. 2018, DOI: 10.1038/s41467-018-04844-y).
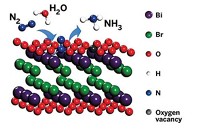
The team’s PEC consists of an electrode made of a light-absorbing semiconductor, p-type indium phosphide, coated with a layer of a rhodium catalyst. When exposed to light, the electrode reduces hydrogen cations from an acidic water solution, producing hydrogen gas.
To simulate the microgravity conditions of space, the researchers catapulted the device 120 m into the air and allowed it to fall in a specialized tower in Germany. During the tests, hydrogen bubbles accumulated on the flat surface of the electrodes, preventing efficient gas production.
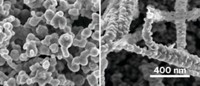
To encourage the bubbles to detach from the electrodes, the researchers wanted to decrease the contact area between the bubbles and catalyst surface, so they shaped the rhodium layer into tiny peaks and holes. When the team tested these textured photoelectrodes in the drop tower, they found the cell’s efficiency matched that of a flat electrode in regular gravity.
Cornell University’s Mason A. Peck, an aerospace engineer and former chief technologist at NASA, said that demonstrating performance in microgravity is an important step for any potential space-faring technology. But ultimately, he notes, the practicality of such a system will depend on how efficient it is at converting solar energy to stored chemical energy. The ideal setup also would produce oxygen. Katharina Brinkert, the paper’s first author, says the team is working to develop a cell that will combine hydrogen and oxygen production.
CORRECTION: The story was updated on July 26, 2018, to correct the caption. The device was tested in a 120-meter drop tube; the tower housing the tube is 146 meters.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter