Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Solar Power
Low-cost polymer works well in perovskite solar cells
New architecture for the devices aids charge-transport process
by Mitch Jacoby
March 27, 2019
| A version of this story appeared in
Volume 97, Issue 13

By modifying the architecture of a perovskite solar cell, researchers have come up with a way to replace a common but costly material used in the devices with a less expensive one (Nature 2019, DOI: 10.1038/s41586-019-1036-3). The advance may hasten the pace of commercialization for these widely studied energy-harvesting devices, which promise cost savings relative to common silicon solar cells.
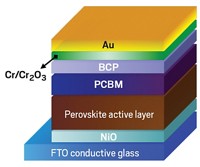
When sunlight shining on a solar cell reaches the photosensitive perovskite layer, typically an alkyl ammonium trihalide, the light excites the material, forming energetic couples consisting of negatively charged electrons paired with positively charged species called holes.
To generate an electric current, the electron-hole pairs must split; electrons go to one of the cell’s electrodes, and holes to the other. The holes reach their destination by traveling through an organic polymer that conducts a positive charge. Nearly all perovskite cells use one of two hole transporters, PTAA and spiro-OMeTAD, both of which are expensive.
Various research groups have tried using a low-cost alternative, poly(3-hexylthiophene) (P3HT), which conducts holes in other organic electronic devices. But invariably P3HT led to poor photovoltaic performance.
“I suspected there was a problem with hole transporting at the interface due to poor contact between P3HT and the perovskite,” says Jangwon Seo of Korea Research Institute of Chemical Technology (KRICT), who led the new study. He thought the holes might run into structural bottlenecks at the interface, allowing the species to recombine with electrons, lose energy, and fail to reach the electrode and generate current. So together with KRICT’s Jun Hong Noh and others, Seo looked for a way to order the structure of the interface between P3HT and the perovskite as a way to boost performance.
Experimenting with an unusual architecture, the group sandwiched a wide-bandgap halide (WBH) material, one that conducts electrons poorly and has long alkyl chains, between a conventional narrow bandgap perovskite and P3HT. They found that the alkyl chains on both sides of the interface interdigitated and helped the materials self-assemble together. Essentially, the WBH improved the contact between the layers and enhanced hole conduction. The new solar cells achieved a near record-setting conversion efficiency of 22.7% and maintained 95% of that level of performance during a 1,370-hr test.
By demonstrating how P3HT can be used as a stable and effective charge-transporting material, this team may have helped accelerate progress in bringing perovskite solar cells to market, says Liyuan Han of Shanghai Jiao Tong University. The results are “truly impressive,” Han remarks, but cautions that follow-up studies must be done to further establish device stability.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter