Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Atmospheric Chemistry
Podcast: How do you solve a problem like the ozone hole?
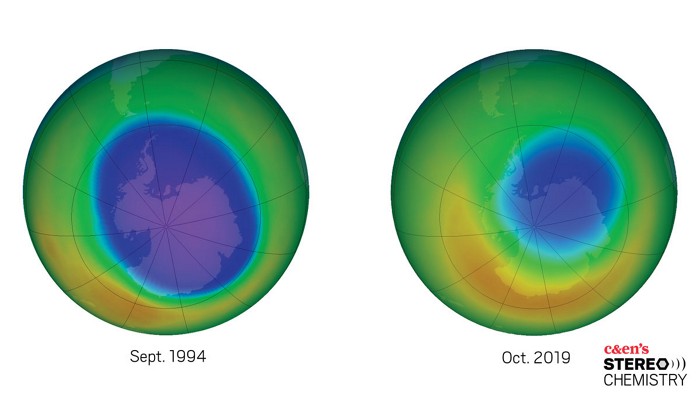
How the world came together to heal the ozone layer—and the lessons learned that can help in the battle against climate change
by Giuliana Viglione
December 17, 2019

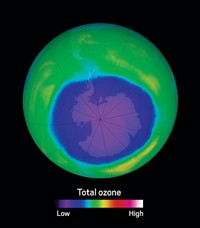
Credit: NASA Ozone Watch | Maps of ozone concentrations above Antarctica from 1979 to 2019 show the gradual healing of the ozone hole.
The discovery of the ozone hole in the mid-1980s sent shock waves through the scientific community and society at large. As scientists scrambled to make sense of the unprecedented phenomenon, a clear culprit emerged. Chlorofluorocarbons (CFCs), once thought of as near-miraculous compounds that revolutionized refrigeration, were suddenly revealed to be one of the biggest environmental dangers known to humankind. What followed was an international push by scientists, media, and policy makers to ban CFCs. In October 2019, NASA announced the ozone hole is the smallest recorded since 1982. In this episode of Stereo Chemistry, we hear from some of the scientists who were instrumental in discovering—and helping heal—the ozone hole and who think lessons learned could help us fight climate change.
To read more from C&EN on hot trends in chemistry, check out our 2019 Year in Chemistry issue at cenm.ag/yic2019.
Subscribe to Stereo Chemistry now on Apple Podcasts, Google Play, or Spotify.
The following is the script for the podcast. We have edited the interviews within for length and clarity.
Susan Strahan: I think we peaked out at about 3.7 ppb of chlorine. But what if we’d gone up to 10, 20, 30? The chlorine levels would have really started eating a hole in the ozone layer. Think of the consequences for crops. Plants are all going to die. If the plants die, we’re all in big trouble. We’re all doomed.
Giuliana Viglione: That was Susan Strahan, an atmospheric scientist at NASA’s Goddard Space Flight Center. She’s talking about the precipitous decline of the ozone layer in the 1980s and ’90s. Susan has spent her career studying the ozone layer that protects our planet. We’ll hear more from her later. But first, we’re going back to the beginning. This is a story about an environmental catastrophe that almost was—and how scientists and policy makers worked together to avert it. I’m Giuliana Viglione, and you’re listening to Stereo Chemistry. I’m joined today by Kerri Jansen, one of C&EN’s multimedia reporters. Hey, Kerri.
Kerri Jansen: Hi, Giuliana. So I get the impression we’re going to be talking about the ozone layer today.
Giuliana: Yes—the invisible defender of planet Earth, way up above us in the stratosphere.
Kerri: So what’s up with the ozone now? I mean, I know there was a hole in it. But we fixed that, right?
Giuliana: It’s sort of a work in progress. Thanks to some pretty forward-thinking environmental regulation in the late ’70s and early ’80s, the ozone hole is on the mend. In fact, in 2019, the ozone hole was the smallest it’s been since 1982. But we’re not quite out of danger yet. Before we get into all that, though, maybe we should go over some fundamentals of atmospheric science.
Kerri: Sounds like a plan.
Giuliana: OK, so the atmosphere is like . . . an onion. The atmosphere has layers. Onions have layers.
Kerri: Are you . . . Are you making a Shrek reference right now?
Giuliana: Look, atmospheres are like onions. End of story. Anyways, the bottom layer—essentially, where all the weather happens—is called the troposphere. The troposphere extends about 10 km into the sky, and above that is the stratosphere. About 90% of the ozone on Earth is in the stratosphere. And since pressure decreases as you go up in altitude, most of that ozone is concentrated in the lower stratosphere. This is what we think of as the ozone layer. Ozone, by the way, is a molecule consisting of three oxygen atoms. Down in the troposphere, it can aggravate respiratory illnesses and damage lungs. But in the stratosphere, it’s a different story.
Kerri: OK, so this layer of concentrated ozone is hanging out in the lower stratosphere. What’s it doing?
Giuliana: It basically acts as the Earth’s sunscreen. The ozone layer absorbs something like 98% of incoming ultraviolet radiation and keeps it from ever reaching the surface. In fact, back when the ozone hole was first discovered, there was a real fear that ozone depletion was going to lead to huge increases in incidences of skin cancer or cataracts, as well as mass die-offs of plants and animals.
Kerri: Oh, so that’s what Susan Strahan was talking about at the beginning of the episode.
Giuliana: Yeah. Not good. Normally, there’s a fine balance between the formation and the destruction of ozone in the stratosphere—a natural steady state that maintains the level of ozone to keep the planet safe. But everything began to change with the invention and popularization of chlorofluorocarbons, or CFCs, in the late 1920s and ’30s.
Kerri: Right, the stuff that was in aerosol cans. CFCs are short-chain hydrocarbons that are at least partially halogenated, meaning some of the hydrogen atoms have been replaced with chlorine and fluorine atoms. And although we think of them as bad chemicals today, they were actually pretty incredible when they first came into use.

Giuliana: Right. As Susan Strahan says, CFCs used to be the good guys. They completely revolutionized cooling and refrigeration, making them much safer and much more widely available. Instead of using ammonia as a coolant, we could use CFCs, which were nontoxic. And as far as anyone could tell at the time, they were inert and completely safe. So we kept manufacturing them and kept pumping them into the atmosphere—tens of thousands of tons per year by the time they began to be phased out.
Kerri: OK, so we’re pouring all of these compounds into the atmosphere, and they’re chemically inert, so they’re just sitting there? That can’t be the end of the story.
Giuliana: Well, in 1973, a young chemist named Mario Molina joined the lab of F. Sherwood—or Sherry—Rowland at the University of California, Irvine, as a postdoc. Sherry had a bunch of different projects available, but Mario was most interested in CFCs and what they might be doing in the atmosphere. Rather than strictly focusing on fundamental chemistry questions, Mario says, he wanted to study something with a potential societal impact.
Mario Molina: These were very stable, very safe chemicals. But we decided anyhow to take a look and see if we could predict what would happen to these chemicals in the environment. So it was a question out of scientific curiosity that led us to explore the consequences.
Giuliana: And in all of his research, Mario couldn’t find any processes in the lower atmosphere that would break down CFCs at all.
Mario Molina: So the conclusion then was that, well, they will eventually find their way into the stratosphere, into the ozone layer or above.
Kerri: And once they get into the stratosphere, they start becoming a problem.
Giuliana: That’s right. As it turns out, the same ozone that protects us from the sun’s UV radiation also protects CFCs in the lower levels of the atmosphere. Once they get into the stratosphere, where ozone can’t protect them as well, they get exposed to more UV radiation, and they can start to break down.
Kerri: And what happens when the CFCs start to break apart?
Giuliana: The ultraviolet light liberates single chlorine atoms from CFCs called chlorine radicals. These radicals have unpaired valence electrons, so they’re incredibly reactive. At first, these solo chlorines react with other compounds to form so-called reservoir gases, like chlorine nitrate and hydrogen chloride. Unlike the radicals, these are pretty stable.
Kerri: Alright, so we’re building reservoirs of chlorine-rich gases in the lower levels of the stratosphere. No problems yet?
Giuliana: Not yet. But as these reservoir compounds keep getting higher and higher in the stratosphere, and they’re exposed to more and more UV radiation, then they can start to break down too.
Kerri: And what does that give you?
Giuliana: More chlorine radicals. And these radicals run amok in the ozone layer. When one of these radicals strikes an ozone molecule, O3, the radical tears away one of ozone’s oxygen atoms. This leaves you with a molecule of diatomic oxygen, or O2, and a chlorine monoxide radical.
Kerri: Another radical. So the reaction continues.
Giuliana: Yeah. When this radical collides with a single, free oxygen atom hanging out in the ozone layer, the result is a second O2 molecule and the original chlorine radical. You’re back to where you started, only with less ozone. As Mario explains:
Mario Molina: Just a single chlorine atom could destroy tens of thousands of ozone molecules because it would be continuously recycled. And so that was what led us to the hypothesis, then, that these industrial compounds, as stable as they were, they could significantly affect the ozone layer.
Giuliana: Although there was a lot of stigma back then about scientists talking to the media, Mario and Sherry Rowland realized that their discovery was too important to keep within the confines of a scientific society. At the 1974 national meeting of the American Chemical Society, Mario and Sherry held a press conference to draw attention to the danger of CFCs. In addition to presenting their scientific results, they called for a complete ban on the substances. They spent the ensuing years continuing to talk to the press and testifying before Congress.
Mario Molina: We decided that it was our responsibility to let society know about this problem, because normally scientists don’t necessarily communicate with the news media, but we thought that there were no environmental organizations dealing with these issues. So we learned about communicating to the media and we also contacted decision makers in government.
Kerri: And were those people interested in listening?
Giuliana: It certainly garnered a lot of attention. DuPont, which was the major manufacturer of CFCs, publicly challenged the conclusions and spent millions of dollars on ad campaigns and lobbying Congress to try and convince the public that CFCs were safe. But despite this pushback, the National Academy of Sciences decided to commission a report to look into the possibility that these chemicals were harming the environment.
Kerri: So the National Academy goes digging. What did they find?
Giuliana: Well, in 1976, just two years after Mario and Sherry published their groundbreaking study, the National Academy released their report, which used models of atmospheric chemistry and transport to unequivocally verify the calculations that Mario and Sherry carried out. At this point, scientists were only making sparse measurements of the ozone layer, but they were becoming more aware of the potential catastrophe that CFCs could bring about.
That same year, the US Environmental Protection Agency announced a phaseout of CFCs as aerosol propellants, a ban that was enacted in 1978. Other countries followed suit, and this work led to the establishment of the Montreal Protocol in 1987, which is pretty widely thought of as the most influential environmental policy ever enacted—it was signed by 197 parties. Here’s Susan Strahan again.
Susan Strahan: All the nations of the world signed on to the Montreal Protocol. Every single one is a signatory. I think that’s really impressive.
Kerri: That does sound impressive. Have other treaties had that level of support?
Giuliana: Well, there was a lot of support for creating the World Health Organization in 1946. And in 1992, for example, the Framework Convention on Climate Change—the precursor to the Kyoto Protocol—had 195 signatories.
Kerri: So I couldn’t help but notice you mentioned climate change in an episode about atmospheric science. Was that foreshadowing, perhaps? Might we hear more about that later?
Giuliana: Oh, for sure. But we’ve got some other important ozone issues to talk about first. It turns out that even Mario Molina and Sherry Rowland didn’t realize the enormity of their discovery yet. See, they thought that the reaction they proposed would lead to a slow erosion of the ozone layer—on the order of 1–2% per decade.
Kerri: Wait. So just the thought of that—1–2% over a decade—was bad enough for all the countries of the world to come together and take action?
Advertisement
Giuliana: Yeah, and remember how I said the CFC-based propellant bans were enacted without a whole lot of direct measurements of ozone? Well, a few years after Mario and Sherry came up with their ozone-depletion hypothesis, scientists at the British Antarctic Survey began to suspect things were actually a lot worse than that 1–2%.
Kerri: How much worse?
Giuliana: I’ll tell you. After a quick break.
Dorea Reeser: Hey there, Dorea Reeser here. I’m C&EN’s audience engagement editor. This is our last Stereo Chemistry episode of the year, so it seemed like a good time to take stock and thank you, our listeners, for following along all year long. This year, you heard stories about underwater robots monitoring our oceans, the innovative chemistries in rocket fuel that never actually made it into rocket fuel, and an in-depth look at the life and legacy of John B. Goodenough, one of this year’s Chemistry Nobel winners. If you’ve got ideas for podcast episodes in 2020, now’s the time to let us know. Email us at cen_multimedia@acs.org with your feedback. That’s cen_multimedia@acs.org. Or if you’re listening to this episode on Apple Podcasts, you can leave a review or rating without even leaving the app. Also, if you’re sitting there sad that 2019 is almost over, never fear. We’re celebrating all the hottest trends and coolest molecules that defined the year for chemists with our annual Year in Chemistry issue. You can check it out at cenm.ag/yic2019. Again, for all the biggest news of 2019 gathered together in one place, go to cenm.ag/yic2019—we’ll post that link in the episode description, too. I could go on and on about the Year in Chemistry review, but it sounds like there’s a whole lot more to cover about the ozone layer. Let’s get back to Giuliana and Kerri.
Giuliana: Before the break, we were talking to Mario Molina, a chemist who won the Nobel Prize for helping show how chlorofluorocarbons, or CFCs, were destroying the ozone layer. And as bad as Mario thought these chemicals were, he didn’t have the full picture.
Kerri: Right. And I’m still waiting for you to tell me how bad the ozone situation actually was.
Giuliana: Well, to understand that, we’re going to need a little lesson about how folks had been keeping an eye on our atmosphere up to this point. Going back to 1957, British scientists had been measuring ozone levels above Antarctica using something called a Dobson spectrophotometer. This instrument measures the relative intensity of UVA and UVB radiation at Earth’s surface and then backs out the total amount of ozone in the atmosphere from those two numbers. The numbers the British scientists were seeing were pretty consistent, with some natural variability. But in the ’70s, those numbers started to drop. And by the early ’80s, the ozone levels were dropping really fast. Way faster than what Mario Molina and Sherry Rowland had predicted. In 1982, the springtime measurements showed that ozone concentration was about 40% lower than its normal value.
Kerri: Wow, 40% loss. That is a lot more than the few percent Mario had thought.
Giuliana: In fact, the levels were so low that Joe Farman, the lead scientist on the project, thought that his instrument was broken. It wasn’t until the next year, when a brand-new instrument showed even more ozone loss, that Farman began to realize that his team was observing a real phenomenon.
Kerri: So what was going on?
Giuliana: Well, while it turns out that CFCs do deplete ozone globally in the way that Mario proposed, there’s something special about the Antarctic that makes the ozone depletion there much, much worse. And that’s how cold it gets—much colder than anywhere else on Earth, even the Arctic. Are you familiar with the term polar night?
Kerri: It sounds like night at the poles.
Giuliana: Solid logic. It refers to the time of year where the sun disappears entirely for months at a time. It gets so cold during polar night that what little moisture exists in the stratosphere freezes into tiny ice crystals. Those crystals can then accumulate in what are called polar stratospheric clouds. These ice crystals also contain a lot of nitric acid, and they form in the lower reaches of the stratosphere.
Kerri: This sounds important, so let me make sure I’m keeping up. During polar night, clouds form in the low stratosphere that are full of ice crystals and nitric acid.
Giuliana: Totally. Now, you remember those reservoir gases I mentioned? While they’re not that reactive in the gas phase in the lower stratosphere, they can react on the surface of these ice particles to create chlorine gas.
Kerri: So these previously inert gases are reacting and creating a buildup of chlorine in the stratosphere?
Giuliana: Yeah. Then, when the sun begins to come up in the Antarctic spring, its UV rays start breaking apart the chlorine molecules to create chlorine radicals, and the ozone depletion begins. This whole process really only happens between August and October—as polar night ends and spring begins. Once the stratosphere starts warming, the ice crystals melt and wind patterns shift, allowing the hole to essentially close up until the next year. This reaction mechanism—the one involving the ice crystals in the polar clouds—was first proposed by Susan Solomon, an atmospheric chemist, then with the National Oceanic and Atmospheric Administration. She led the 1986 US Antarctic expedition that helped confirm this mechanism.
Kerri: But 40% is a huge amount of ozone to go missing. Didn’t anyone else notice?
Giuliana: There were other groups looking at ozone at the time. NASA’s Nimbus 7 satellite, launched in 1978, carried an instrument called TOMS—the Total Ozone Mapping Spectrometer. This satellite was orbiting Earth and creating global maps of ozone, but there was a problem. You see, the ozone levels over Antarctica dropped so low during Antarctic spring in the 1980s that the instrument wasn’t calibrated for these values. So those data were flagged as bad and rejected.
Kerri: They just threw the data out?
Giuliana: Well, no, but it wasn’t included in the ozone maps they were making. Eventually, NASA scientists realized that more and more data were falling below the quality-control level, but they weren’t sure what the problem was. Since they didn’t have any other data to compare theirs to, they weren’t sure if they could trust the numbers they were seeing.
Kerri: But weren’t the Brits measuring ozone levels since the late 1950s?
Giuliana: Yeah, and some Japanese scientists in Antarctica were making their own measurements, too. But none of this information was really being shared around. It wasn’t until the Brits published their findings in 1985 that people were able to compare the ground-based observations with the satellite data. When NASA went back and reprocessed their flagged data, it revealed the true extent of the ozone hole—an area of low ozone concentrations roughly the size of the continental United States.
Kerri: So maybe we could have been working to solve the ozone problem sooner if folks had been more forthcoming with their data?
Giuliana: There’s actually still a lot of contention about how much NASA knew about the ozone hole and when they knew it. But whatever you believe, the discovery of the ozone hole certainly galvanized the world into action.
Mario Molina: The case became stronger when the ozone hole over Antarctica was found because that’s not something we had initially predicted. But we and others then suggested, “Yeah, this is also caused by these industrial chemicals,” and then further scientific research was carried out that proved rather unambiguously that indeed we were correct.
Kerri: What sort of research is he talking about?

Giuliana: He’s referring to the Airborne Antarctic Ozone Experiment, which was a massive field campaign that took place in August and September of 1987. It involved two aircraft decked out with a suite of scientific sensors, 25 total flights, and something like 200 scientists looking at the data. Basically, there were a bunch of prevailing theories about why the ozone hole formed and why it went away and came back every year. To put those theories to the test, all of these scientists descended on Punta Arenas, the southernmost city in Chile, to try and collect the data needed to settle things.
Susan Strahan was a year away from completing her PhD in chemistry when she first heard about the ozone hole. Having spent her PhD working on instrumentation, she was attracted to the idea of tackling a massive problem with real societal implications. She started working on airborne missions as a postdoc at NASA’s Ames Research Center and soon found herself heading down south as part of the ozone experiment.
Susan Strahan: The aircraft would go up every couple of days, and then they would come back, we’d download the data. And then it was really exciting.
Giuliana: This was 1987, before you could just plot something in Excel and email it to your adviser or your coworkers. Susan said the researchers were charting their data and pinning it to bulletin boards in the airplane hangars at the Punta Arenas airport. So you had all these scientists testing different theories, plotting the concentrations of everything from chlorine monoxide radicals to nitrous oxide, but pinning the plots to the same boards, then standing around and comparing notes with each other.
Susan Strahan: And then we’re seeing this all at the same time going, “Huh? OK, so what if we, you know, we plot these things together? What is that telling us? These things are correlated. What does it mean?” And we were just doing the science real time.

Giuliana: She says it was from this collaboration that they were able to develop “smoking gun” plots that really convinced the world that CFCs were causing the ozone hole. She also describes the collaborative nature of the field campaign as a truly formative experience for an emerging scientist.
Susan Strahan: We were just excited that we were getting the answers. I think this drives a lot of scientists is that we’re doing this stuff, we’re doing whatever research we do, because it’s curiosity-driven research. And so here’s this big phenomenon, and there’s also this sense of we need to know the answer. This is really important. This matters to all of us.
Kerri: Wait, but if Susan Strahan was making her measurements in 1987, that means the Montreal Protocol was ratified before we knew that CFCs were causing the ozone hole?
Giuliana: Actually, yes. The work to put the Montreal Protocol into place began as a way to put an end to the kind of slow ozone depletion that Sherry Rowland and Mario Molina proposed. At the time, scientists thought it was possible—or even pretty likely—that CFCs were playing a role in the formation of the ozone hole, but they weren’t totally sure if the two were linked. It wasn’t until the results of the Airborne Antarctic Ozone Experiment were published that the connection was firmly established.
Advertisement
Kerri: At which point the process to phase out CFCs had already begun.
Giuliana: Right.
Kerri: So what’s the status of the ozone hole now?
Giuliana: It’s finally beginning to heal after almost a century of our emitting these ozone-depleting compounds. Mario Molina, Sherry Rowland, and Paul Crutzen were jointly awarded the 1995 Nobel Prize in Chemistry for their work showing how CFCs and other industrial chemicals were depleting stratospheric ozone—the first time the prize had been awarded for environmental science. And in late October 2019, NASA announced that the ozone hole was the smallest it’s been since 1982, the first year that the hole appeared.
Kerri: Wow! That’s great news, right?
Giuliana: Well, sort of. Because the ozone-depletion reaction in the Antarctic is dependent on the presence of those tiny ice particles in the stratosphere, the size of the hole is controlled by the stratospheric temperature. This year happened to be an exceptionally warm year, so the hole was pretty small. So, NASA’s atmospheric models do show us that the ozone hole is getting better compared to what it would have been if we’d kept emitting CFCs, but this wasn’t a magical recovery year—it’s just natural variability. The best estimate says that the ozone hole should be fully recovered by the end of this century.
Susan Strahan: The road to recovery is really bumpy. You know, we’re going downhill like in a Jeep going down a mountainside. And sometimes, you know, it’s a really bumpy road and you go up some, you go down some, and wow, are we really going down? We are. But with the ozone hole, if you just look at the changes from one year to the next, you can’t tell. But if you wait and you look at decade-over-decade changes, then you start to see a trend.
Giuliana: And some of the bumps along the way are caused by humans, too. A few years ago, some scientists realized that the concentrations of CFCs in the atmosphere weren’t declining as much as they expected. In fact, the rate of decline had slowed to half of what it should have been.
Kerri: Somebody was making CFCs again?
Giuliana: That’s right. Eventually, researchers were able to trace 40–60% of these “fugitive emissions” back to provinces in eastern China. The Chinese government and an international organization called the Environmental Investigation Agency have begun to crack down on these manufacturers. So despite the immense success of the Montreal Protocol and the ensuing healing of the ozone hole, we have to be vigilant.
Susan Strahan: Even though we think of the ozone hole, or the ozone layer, as sort of a solved problem—because we did come up with an effective solution—this was a reminder that that solution is only effective as long as everybody plays by the rules.
Giuliana: I asked Susan if she saw any commonalities between the fight to ban CFCs in the 1970s and ’80s and the ongoing fight against greenhouse gas emissions and climate change.
Susan Strahan: Oh, heck yeah. [Laughter] Definitely.
Giuliana: And I posed the same question to Mario Molina.
Mario Molina: What is common to these two problems is they are both global—namely, they are consequences of the emission of certain compounds, gases, that remain for quite some time in the atmosphere. So it doesn’t matter where they are emitted. You cannot solve climate change or stratospheric ozone just by stopping emissions in a few countries. You have to do it all over the planet.
Giuliana: As an aside, because CFCs are also very potent greenhouse gases, the Montreal Protocol is the global policy that has had the biggest impact on slowing climate change.
Kerri: Oh, nice. So it’s a regulatory twofer. It shrinks the ozone hole and it slows warming. So you really have to wonder, then, What’s so different about CFCs? How did the world get on board with banning them and then show so much reluctance to do the same with greenhouse gas emissions?
Giuliana: Well, CFCs were important, but it was relatively easy to replace them in most uses with things like hydrofluorocarbons, which don’t contain any chlorine and break down more easily in the lower atmosphere. Burning fossil fuels is much more central to our entire way of life. And ozone depletion happened much more quickly than climate change, which made it harder to ignore.
Susan Strahan: The ozone hole was a really dramatic phenomenon. And it really captured people’s attention. It was like, bam, half the ozone layer over Antarctica is gone and comes back later, but every year it goes away. And you know that that’s really shocking. Climate change—it doesn’t hit you over the head as much because there’s a lot of natural variability. It makes it a lot harder to get everybody on board.
Kerri: So what do these scientists think we should do about it?
Giuliana: Well, Susan Strahan was careful to say that evidence-based policy making requires really good, unambiguous, unbiased science. It’s up to the scientists to come up with the data and present it clearly, but eventually, it’s going to be on us—all of us—to act. And Mario Molina said something similar.
Mario Molina: Science doesn’t tell us what to do. Science can only tell us what will happen if society does certain things. It is actually society—it’s an ethical issue, a moral issue whether to deal with these problems.
Giuliana: But if there’s one takeaway message that both of them wanted me to have, it’s a message of hope. Ozone depletion was a real, potentially catastrophic issue, and the world came together to solve it. They think we can do the same thing with climate change, even if it’s a more difficult problem in a lot of ways.
Susan Strahan: The big lesson is science and policy can work together to solve big problems. Ozone destruction was an existential threat. Scientists did their science, they wrote up a big consensus report to show to policy makers around the world. And policy makers worked with them and said, “OK, here’s the policies we can agree on. We can implement these and go forward to solve this problem.” So, it can be done. I think the climate change problem is harder. It’s more complex. And it’s—some people are harder to convince, even though the data is very overwhelming. I’m optimistic that we’ll work it out because we know it can be done.
Giuliana:Stereo Chemistry is a production of C&EN, the news magazine of the American Chemical Society. Tune in next month for another exciting episode. You can subscribe on Apple Podcasts, Google Play, Spotify, or wherever you listen to podcasts. This episode was written by me, Giuliana Viglione, and produced by me and Kerri Jansen. It was edited by Matt Davenport, Lauren Wolf, and Amanda Yarnell. Sabrina Ashwell is our copyeditor.
Advertisement
Kerri: Our intro music was “New Land,” and right now you’re hearing “Breathe,” both by Ian Post. The music during our promo was “Verve” by Assaf Ayalon.
Giuliana: Thanks for listening!
Fin.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter