Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Green Chemistry
Computing a cleaner polyurethane synthesis
Advanced modeling identifies an efficient catalyst that experiments show can make polyurethanes without unwanted formaldehyde emissions
by Tien Nguyen, special to C&EN
June 12, 2019
| A version of this story appeared in
Volume 97, Issue 24

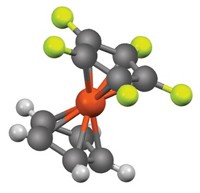
Polyurethanes, found in many everyday products including footwear and furniture, possess enviable properties, but their synthesis leaves more to be desired. Manufacturers use reactive N,N-dimethyl amine catalysts that are incorporated into the material and give off toxic formaldehyde over the products’ lifetimes, a particular concern for polyurethanes used indoors. Now, Mikko Muuronen, Peter Deglmann, and Željko Tomovićat of BASF have used a computational model and experiments to evaluate the activity of alternative amines, identifying catalyst candidates that avoid the toxic by-product (J. Org. Chem. 2019, DOI: 10.1021/acs.joc.9b01319).
Manufacturers produced over 16 million metric tons of polyurethanes in 2015 via the reaction between an aromatic isocyanate compound and a polyol catalyzed by a tertiary amine. The methyl groups in the standard N,N-dimethyl amine catalysts can break down, and formaldehyde is one of the degradation products. The researchers thought that alternative amines lacking methyl substituents could avoid this issue. The team modeled this reaction with the N,N-dimethyl amine and six alternative amine catalysts with longer alkyl substituents and various ring sizes to determine the activation free energy in the transition state—a measure of the catalyst’s activity.
The team confirmed their calculations in experiments, identifying that the two catalysts with the lowest free energy also performed the best and observing that catalysts with larger rings and electronegative substituents had less activity. Then, the team prepared polyurethane foams from the two most active catalysts, a tertiary N,N-dimethylamine and a tertiary pyrrolidine compound. Foam made from the former gave off high levels of formaldehyde whereas no formaldehyde was detected from foam made with the latter.
The work shows that quantum chemical computations can allow researchers to predict catalytic reaction behavior, says Minh Nguyen, a physical chemist at KU Leuven, adding that it is interesting that the team was able to confirm their own predictions with synthetic experiments. BASF declined to say whether any of these catalysts are being pursued for further development and use in manufacturing processes.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter