Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Green Chemistry
Electrochemical Haber-Bosch cuts energy demand and CO2 emissions
Shrinking the multistep process into 1 reactor could make the critical industrial process more sustainable
by Leigh Krietsch Boerner
November 7, 2019
| A version of this story appeared in
Volume 97, Issue 44
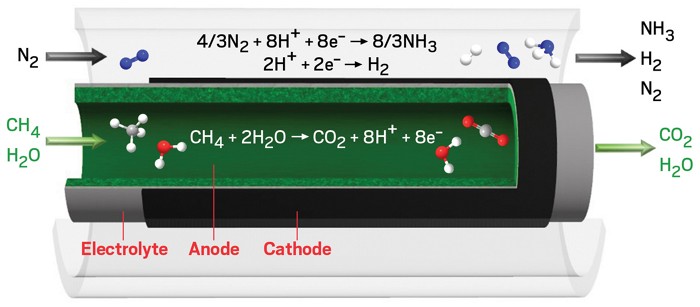
The Haber-Bosch process is responsible for over 1% of global carbon dioxide emissions every year, making it the biggest CO2 belcher among industrial chemical reactions. The multistep reaction, which creates ammonia out of nitrogen and hydrogen gases at high pressures and temperatures, feeds the agricultural industry with enough fertilizer to produce over 50% of the world’s food. Now Michael Stoukides and coworkers at Aristotle University of Thessaloniki have reduced Haber-Bosch to a single step run at atmospheric pressure using an electrochemical reaction in a BaZrO3-based protonic ceramic membrane reactor (Joule 2019, DOI: 10.1016/j.joule.2019.10.006). Under ideal conditions, the approach uses 25% less energy and spits out 50% less CO2 than traditional Haber-Bosch systems.
The key to consolidating the process into a single step was bringing all the separate reactions close together. Stoukides and his colleagues created a system with a BaZrO3 electrolyte sandwiched between a vanadium nitride–iron cathode and a nickel-based anode.
This strategy has two main advantages, says Vasileios Kyriakou, first author on the paper. Much of the energy consumed in a traditional Haber-Bosch process is used during the high-temperature steam reforming of CH4 to make hydrogen gas, as the reaction runs at around 1,000 °C. In the new system, CH4 and steam are converted to CO2, H+ ions, and electrons at the nickel anode. Only the H+ ions are allowed to pass through the solid electrolyte to the cathode. By removing the ions from the anode, the system increases CH4 conversion due to the Le Chatelier principle. When the H+ions cross into the cathode, they react with N2 to form NH3. In industrial Haber-Bosch reactions, H2 and N2 gases get combined at very high pressures to activate the strong N-N triple bond. The new system’s second advantage is that the N2 is activated electrochemically at the cathode to combine with H+.
While this system still uses a carbon input, it reduces the CO2output, which could make it a viable, more sustainable option in the near term, says Karthish Manthiram, a chemical engineer at the Massachusetts Institute of Technology.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter