Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Persistent Pollutants
The hunt is on for GenX chemicals in people
Analysis of North Carolina residents’ blood for Chemours PFAS yields surprises
by Cheryl Hogue
April 7, 2019
| A version of this story appeared in
Volume 97, Issue 14

On a chilly day in November 2017, Beth Markesino sat in the county health department. She pushed up the sleeve of her sweater and extended her arm. A medical technician drew her blood. During her visit to the facility, Markesino spotted several people she knew and said hi to them. They were getting blood work done too.
They hadn’t come for routine medical tests. Markesino and her neighbors in Wilmington, North Carolina, were giving blood samples so researchers could analyze them for more than a dozen fluorochemicals released by a Chemours plant 100 km away. Scientists had found a cocktail of unregulated industrial chemicals in the Cape Fear River, the source of their public drinking water. Scientists in 2016 reported finding per- and polyfluoroalkyl substances (PFAS) in that drinking water, despite it being treated by a local utility (Environ. Sci. Technol. Lett. 2016, DOI: 10.1021/acs.estlett.6b00398).
Markesino and others assembling at the health department in 2017 are among the more than 300 residents of the Wilmington area who volunteered to give blood and urine samples as part of a PFAS exposure study. North Carolina State University researchers are leading that investigation.
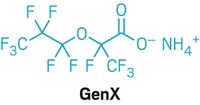
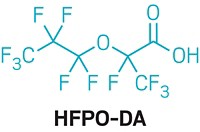
Many volunteers, including Markesino, were particularly worried about a chemical called GenX, which Chemours makes, then uses as a processing aid in the manufacture of fluoropolymers for nonstick coatings and other applications. Scientists found that in water, the chemical GenX hydrolyzes into hexafluoropropylene oxide dimer acid (HFPO-DA), which was among the PFAS found in the treated drinking water.
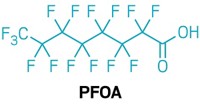
GenX was introduced in 2009. Its inventor, DuPont, called it a “sustainable replacement” for the persistent, bioaccumulative, and toxic chemical perfluorooctanoic acid (PFOA), which the company formerly used as a fluoropolymer processing aid. When DuPont spun off its fluorochemical business to form Chemours in 2015, the new company continued to make and use GenX at a factory outside Fayetteville, North Carolina. A growing body of studies suggests that HFPO-DA, like the well-studied PFOA, is linked to harmful effects in the liver and reproductive problems (Environ. Health Perspect. 2019, DOI: 10.1289/EHP4372).
After the Wilmington newspaper the StarNews published a series of articles about the PFAS contamination in June 2017, Markesino sprang into action. She formed a Facebook group of Wilmington residents demanding clean drinking water. That group has since grown to include people across the US and in Australia living in other communities that face PFAS contamination. Markesino is now president of a nonprofit advocacy organization called North Carolina Stop Gen-X In Our Water. The group shares information about PFAS found in drinking water and, through its members’ donations, has sponsored Wilmington-area billboards imploring Chemours, “Stop polluting our air, water and soil with your toxic chemicals.”
Like many of their Wilmington neighbors, Markesino, her husband, and their now 6-year-old daughter stopped drinking their tap water in June 2017. The family now cooks, brews coffee, and brushes teeth with bottled water, Markesino tells C&EN. Her daughter carries two reusable bottles containing filtered water to school each day. Markesino has instructed her not to drink the school’s water.
Markesino says her advocacy work and changing the source of the water her family consumed were empowering steps after the shock of learning about the PFAS contamination. But in the following months, worries increasingly gnawed at her. She thought about the training she’d done as a marathon runner, when she kept hydrated by sucking down liter after liter of what she now knows was tainted Wilmington tap water. And she grieved over Samuel, the baby she gave birth to in her 24th week of pregnancy in October 2016. She wondered if her son’s death from kidney, bladder, and bowel malformation was connected to the PFAS in the water she drank.
“I’ll never know what was in Samuel,” Markesino says. But when NC State researchers sought volunteers for the exposure study, she signed up to find out about the PFAS in her own body.
After providing their blood and urine samples in late 2017, Markesino and the other volunteers in the GenX Exposure Study began waiting for the results. A team of researchers from NC State and East Carolina University analyzed the samples for 23 PFAS chemicals using mass spectrometry. These chemicals included HFPO-DA; by-products from the Fayetteville plant’s operations that have been found in the Cape Fear River; fluorotelomers that are ingredients in some firefighting foams; and so-called legacy PFAS, including PFOA, that are no longer made in the US.
Months passed.
In May 2018, Markesino joined 43 other study participants who volunteered for a second blood draw. The results would allow researchers to assess how long any PFAS found in the first samples stayed in their blood.
.jpg)
More months passed.
Meanwhile, in June 2018, a team led by Zachary R. Hopkins, a doctoral engineering student at NC State, published analyses of PFAS found in Cape Fear River water and in drinking water treated by the Wilmington utility during 2017 (J.—Am. Water Works Assoc. 2018, DOI: 10.1002/awwa.1073). The researchers detected a suite of fluorinated chemicals, some not identified in previous papers. They also found that the concentration of PFAS dropped sharply after Chemours began to capture HFPO-DA-containing process wastewater instead of discharging it into the river.
Finally, in November 2018, volunteers got envelopes in the mail with their blood test results. While individual data are confidential, the participants’ combined results are public.
First, the study found no detectable HFPO-DA—the GenX chemical—in participants’ blood.
Markesino felt relief. The GenX chemical “was the thing that everyone was so concerned about,” she says.
“Because we could not detect GenX in blood samples, we know that GenX does not stay in the blood for very long,” says a letter that participants received from the study’s principal investigator, Jane Hoppin, an NC State environmental epidemiologist.
The study’s limit of detection for this substance was 2 ppb in blood. In contrast, researchers routinely measure PFAS in water in parts per trillion.
Also, the analysis showed the volunteers’ blood had no detectable amounts of two PFAS that are widely found in the environment: perfluorohexanoic acid, a breakdown product of stain- and grease-resistant coatings, and 6:2 fluorotelomer sulfonate, an ingredient in many aqueous film-forming foams used to fight liquid fuel fires and in coatings on food wrappers.
That was the good news.
The tests showed that Markesino and the other volunteers, like most people in the US, have PFOA and four other historically used PFAS in their blood. Wilmington-area residents in the study had 4.4 ppb of PFOA in their blood drawn in late 2017, while the US average was 1.5 ppb in the Centers for Disease Control and Prevention’s 2013–14 National Health and Nutrition Examination Survey.
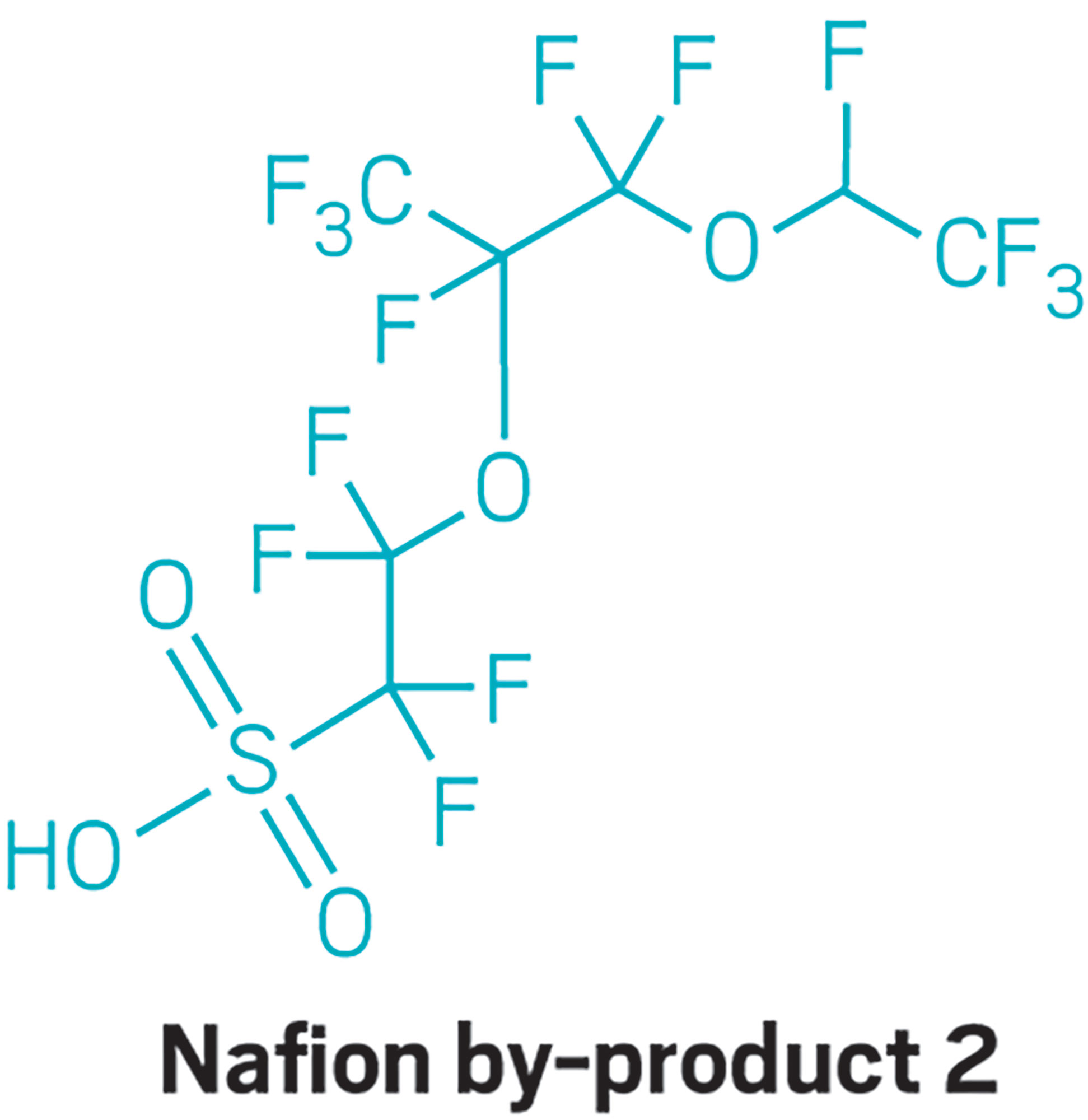
In addition, the results found that volunteers’ bodies harbor four PFAS that scientists know little about. Chemours tells C&EN that all four of these substances are manufacturing by-products that are not produced for commercial purposes.
One of these four, which researchers call Nafion by-product 2, was in 99% of the blood samples analyzed. This chemical is a by-product of making Nafion, Chemours’s sulfonated tetrafluoroethylene-based ionic polymers that are used for membranes in fuel cells, chlor-alkali production, and other applications. Nafion by-product 2 is a multiether sulfonic acid.
Participants’ median blood concentration for Nafion by-product 2 was more than 2.5 ppb, the blood analysis found. The blood samples also contained the carboxylic acid form of Nafion by-product 2, an ester vinyl ether (EVE) called hydro-EVE acid.
Hopkins’s NC State group also found Nafion by-product 2 in water samples. The water analysis team detected but did not report the presence of hydro-EVE acid in its peer-reviewed results, says Detlef Knappe, an NC State engineering professor and Hopkins’s PhD adviser.
“We did not yet know what it was, and we did not have an analytical standard for it,” Knappe tells C&EN. “But it was present in the water.”
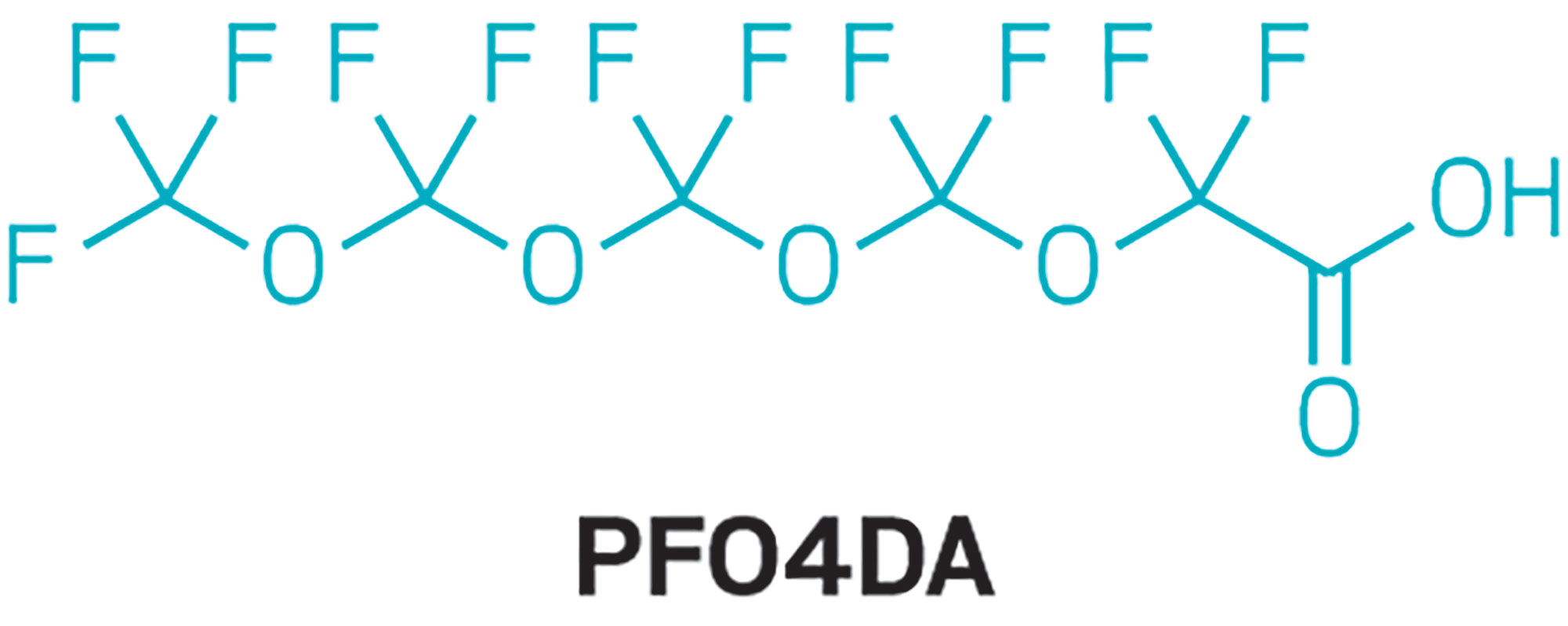
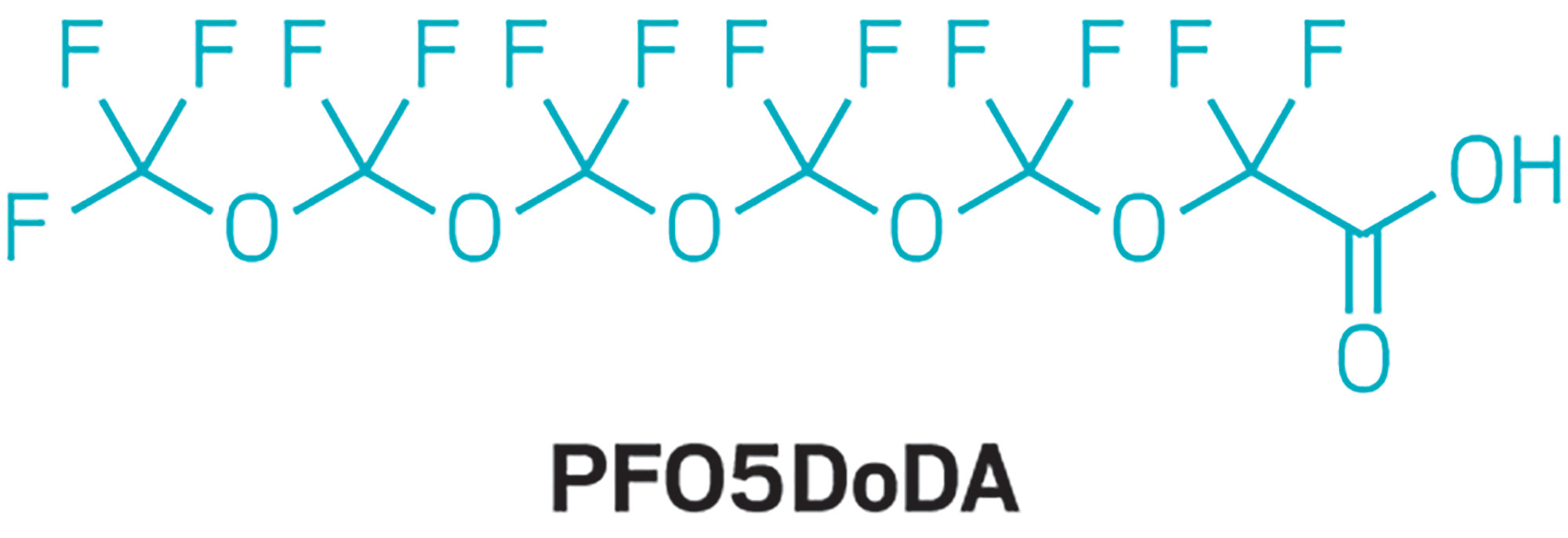
Epidemiologist Hoppin’s team also found two other novel fluoroethers in the blood samples. Perfluoro-3,5,7,9-tetraoxadecanoic acid (PFO4DA) was in 98% of the samples. Perfluoro-3,5,7,9,11-pentaoxadodecanoic acid (PFO5DoDA) was found in 87% of the samples. Volunteers’ median blood concentrations were more than 2.0 ppb for PFO4DA and less than 0.5 ppb for PFO5DoDA.
At least one of those two perfluorinated chemicals was in the water too, Knappe says. “PFO5DoDA was likely present in the water at concentrations below our reporting limit.” His lab is working to make its analytical method more sensitive in the hope of detecting this chemical, especially in older, stored samples of Cape Fear River water.
A few weeks before Markesino and the other volunteers gave their first blood samples, Chemours started capturing wastewater from its Nafion manufacturing processes. The company adopted this practice because North Carolina regulators revoked the plant’s wastewater discharge permit for fluoroether production. The chemical maker now hauls this wastewater to Texas for disposal via deep-well injection, Chemours told North Carolina and the US Environmental Protection Agency in 2017.
After Chemours stopped discharging wastewater to the river, Hopkins’s team saw levels of PFAS, including the Nafion by-product, drop sharply in the water—although they didn’t disappear entirely. This suggests these chemicals may be leaching out of river sediment or transported through stormwater runoff or contaminated groundwater, the team’s paper says.
Meanwhile, Hoppin’s team found that median blood levels of Nafion by-product 2, PFO4DA, and PFO5DoDA dropped in the 44 participants, including Markesino, who had their blood retested in May 2018.
Markesino says she is not surprised that fluorochemicals that she’d never heard of turned up in her blood. Instead, she says, “I’m surprised that the scientists knew how to detect them” in blood.
She’s indignant that the novel industrial contamination was imposed on her family and her community without their knowledge or consent.
Advertisement
“This is in our river. It’s in my body,” she says of the PFAS pollution. “It’s in our rain. It’s in our dust. It’s in our soil.” She blames Chemours and its predecessor, DuPont; North Carolina regulators; and the US Environmental Protection Agency for the contamination in the Cape Fear River and, in turn, her and her neighbors.
What the blood test results portend for the health of Markesino and her neighbors is a looming question. When the results were mailed out, there were no published health or toxicology data for Nafion by-product 2, hydro-EVE acid, PFO4DA, or PFO5DoDA.
“Therefore, we cannot say what these results mean for your health,” says Hoppin’s letter to participants.
“It scares me,” Markesino says of not knowing whether the levels of PFAS in her body are safe or pose a risk to her current and future heath.
Now, new data suggest her fear may have a scientific basis, at least for PFO4DA in mice. In March, a team led by Hua Guo of the Chinese Academy of Sciences published what the authors believe is the first study of the toxic effects of perfluoroether carboxylic acids, including PFO4DA. Their 28-day study in male mice showed PFO4DA caused harmful effects to the liver (Environ. Sci. Technol. 2019, DOI: 10.1021/acs.est.9b00148).
“Efforts to remove or at least decrease its [PFO4DA’s] occurrence in drinking water should be made urgently,” Guo and colleagues write in their paper.
Chemours says an independent laboratory has tested PFO4DA and PFO5DoDA along with eight other “PFAS waste substances” and found none displayed the potential to cause genetic mutations. A chemical’s ability to alter genetic material is often linked to carcinogenicity. Chemours says it is committed to further testing of these and other PFAS.
Meanwhile, researchers in the exposure study are still analyzing urine samples that Markesino and her fellow study participants provided.
“I’m very anxious to see how those come back,” she says.
CORRECTION:
This story was updated on April 10, 2019, to correct the structure for hydro-EVE acid. Because of a production error, it appeared as an aldehyde rather than an acid.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter