Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pesticides
Computationally designed enzyme inhibitors overcome pesticide resistance
The boronic acids could cut insecticide usage by 90%, study authors claim
by Giuliana Viglione
October 11, 2019

Scientists have documented insecticide resistance in more than 580 insect species since the 1940s, leading to increasing concerns that this loss of effectiveness will threaten both public health and food security. But a new study suggests that boronic acids that target the enzymes pests use to counteract insecticides may act synergistically with the insecticides to overcome resistance (Proc. Natl. Acad. Sci. U.S.A. 2019, DOI: 10.1073/pnas.1909130116).
The researchers combined both computational and experimental techniques and found that using a boronic acid in conjunction with a traditional insecticide increased the effectiveness of several common insecticides up to 16-fold. They think that using their newly identified synergists could decrease pesticide use by 90%.
Organophosphates and carbamates, two of the most widely used classes of insecticides, work by inhibiting a key enzyme, acetylcholinesterase. The result is a buildup of the neurotransmitter acetylcholine, which then causes convulsions, paralysis, and, eventually, death. However, more than 50 insect species have developed resistance to this mode of action. In these species, mutated enzymes called carboxylesterases intercept the pesticides and sequester them or break them down to stop them from acting on acetylcholinesterase.
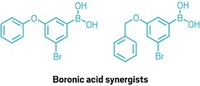
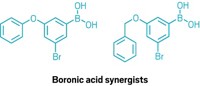
Rather than searching for a new type of insecticide, the team, led by University of California, San Francisco structural biologist Galen Correy, focused on identifying a molecule that could inhibit those carboxylesterases. Correy conducted the research while working towards his PhD at the Australian National University. The scientists looked at boronic acids because they are known to bind with high affinity to other enzymes with similar binding sites. They used a computational method to screen a virtual library of more than 23,000 boronic acids for their covalent binding affinity to a chosen carboxylesterase. After inspecting the 500 top-performing compounds and throwing out those that weren’t easily purchasable or didn’t appear reasonable, the team landed on five structurally diverse candidates.
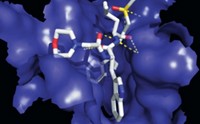
The researchers validated their computational results by obtaining crystal structures of the boronic acid-carboxylesterase complexes for the five compounds and comparing them to those predicted by their software. From these five selected compounds, the team chose the two that bound most potently to both the wild-type and mutated carboxylesterases. Then, they looked for commercially available compounds that combined the structural features of those two molecules.
The most promising compounds were tested against two major pests, sheep blowfly larva and adult peach-potato aphids. The inhibitors amplified the killing power of several common organophosphates against both species, including an “almost total extermination” of the pesticide-resistant aphid population.
The team acknowledges that the use of any inhibitor is unlikely to be a permanent solution. After all, “resistance issues are going to be present with any biochemical target,” Correy says. “You can’t avoid it.” However, he adds, any mutations that would render the boronic acid synergist ineffective would hopefully also negate the original mechanism of resistance.
The researchers also tested several of the most promising compounds for toxicity in human cells and live mice; high doses were fully tolerated in mice and showed low toxicity for human cells. However, further safety testing is required to make sure there are no off-target synergistic effects between the inhibitors and the pesticides, says Nsa Dada, a research entomologist at the Norwegian University of Life Sciences. Dada praises the group’s virtual screening approach, saying it could make the process of finding new compounds in the fight against insecticide resistance “a lot faster than we’re used to.”
Carol Parish, a computational chemist at the University of Richmond, says the study was a “pretty exciting use” of covalent docking simulation, a method that has started to garner a lot of interest over the past few years. She also praises the team’s multifaceted approach. The work, she says, is “a very nice example of the blending of computational and experimental work.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter