Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pollution
Improved version of oxygen-storage material boosts engine-emission cleanup
Simple catalyst treatment could lead to better catalytic converters
by Mitch Jacoby
September 19, 2020
| A version of this story appeared in
Volume 98, Issue 36
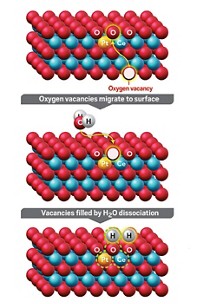
A simple heat treatment can alter the structure and thermal stability of a cerium-zirconium material, thereby improving its ability to strip pollutants from automobile engine emissions (Appl. Catal., B 2020, DOI: 10.1016/j.apcatb.2020.119450). Catalytic converters on gasoline-powered vehicles scrub toxic combustion products in the exhaust stream with three-way catalysts (TWCs). The name reflects the catalysts’ ability to oxidize carbon monoxide and hydrocarbons and reduce smog-forming nitrogen oxides. The key to TWCs’ knack for mediating oxidations and reductions—chemically opposed reactions—is a rhodium-doped cerium oxide–zirconium oxide (CZO) oxygen-storage material. CZO intermittently stores oxygen during reduction steps and releases it during oxidations. Today’s TWCs use the tetragonal form, t-CZO. Recent studies suggest that the pyrochlore form, pyr-CZO, which has a different lattice structure, could outperform t-CZO. But pyr-CZO morphs into t-CZO at high temperatures, and it has low surface area, rendering it ineffective catalytically. A research team including Jason Wu of Ford Motor and Joerg R. Jinschek of the Ohio State University has shown that treating commercial CZO with hydrogen at 1,200 °C followed by a simple cooling procedure leads to high-surface-area pyr-CZO, which retains its structure after aging at 910 °C. And it outperforms t-CZO in converting nitric oxide and hydrocarbons.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter