Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Water
Wastewater from fracking: Growing disposal challenge or untapped resource?
Question looms large as US regulators consider weakening discharge rules to help manage increasing volumes of produced water
by Britt E. Erickson
November 17, 2019
| A version of this story appeared in
Volume 97, Issue 45

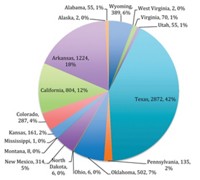
Natural gas production in the US is at an all-time high, according to the latest reports from the US Energy Information Administration. But the dramatic growth of shale gas over the past decade, made possible by hydraulic fracturing, or fracking, has led to huge volumes of salty wastewater called brine or produced water.
As the fracking industry improves its efficiency by drilling ever-longer horizontal wells, it also increases the amount of water it uses to fracture the rock to release the gas. The fracturing process uses on average about 45 million L of water for a single horizontal well, according to the Groundwater Protection Council (GWPC), a group of state oil and gas regulators and environmental protection agencies.
Water pumped into fracking wells doesn’t all stay in the ground. Much of it comes back up along with extracted gas. The water that comes up has a much different chemistry than the water that goes down. Produced water typically includes salts from dissolution of the underlying rock, naturally occurring radioactive substances, and chemicals added during the drilling and fracking process.
It is unclear exactly how much produced water the fracking industry generates. It is also difficult to characterize the chemical composition of produced water because many of the chemicals used by the fracking industry are proprietary, and the geology of natural gas formations varies widely across the US.
The GWPC estimates that the US oil and gas industry generates approximately 3,400 billion L of produced water each year. That number is based on data from nearly 1 million oil and gas wells, which are increasingly hard to tease apart because many wells extract both oil and gas. The US Environmental Protection Agency predicts that volumes of produced water from the fracking industry will continue to increase as natural gas production rises.
The EPA currently prohibits the discharge of produced water to surface waters and municipal wastewater treatment plants, with one exception. Companies may discharge produced water west of the 98th meridian—which runs through eastern North Dakota down through eastern Texas—if the water quality is good enough for agriculture or wildlife and is actually used for such purposes. It is unclear, however, what “good enough” means.
Most produced water is disposed of by injecting it into deep underground wells, but the geology in some parts of the US is not amenable to such practices, and concerns about inducing seismic activity have the industry looking for alternatives. Increasingly, the industry is reusing produced water for drilling and fracking. In some areas where produced water is less salty, primarily in Wyoming, farmers use it to irrigate crops or provide water for livestock. A handful of water treatment facilities that can handle industrial waste are also now treating produced water.
The EPA is considering changing its policy to give the fracking industry more options to discharge produced water. The agency released a draft document in May summarizing practices used by oil and gas companies to manage produced water. The document also provides a snapshot of the pros and cons of allowing more discharges of produced water.
What is in produced water?
One of the biggest concerns is the lack of data on the chemical composition of produced water. To help fill that gap, a team of researchers at West Virginia University is characterizing produced water from fracking wells in West Virginia as part of a multiyear study funded by the federal Department of Energy. Called the Marcellus Shale Energy and Environment Laboratory (MSEEL), the study aims to reduce the environmental impacts of fracking and improve the efficiency of natural gas recovery.
From earlier work analyzing produced water from fracking wells in Pennsylvania and West Virginia, the WVU team had some idea of the chemical composition of the water. Common elements found in produced water include cations of the metals sodium, magnesium, calcium, strontium, and barium; the transition metals manganese and iron; and aluminum, arsenic, and selenium, says Paul Ziemkiewicz, director of the West Virginia Water Research Institute at WVU and a leader of the MSEEL effort to characterize produced water. Produced water also contains the anions sulfate, chloride, and bromide, he says.
Predicting how much of each ion is present in produced water is difficult because the concentrations are “all over the place” depending on when a water sample is taken, Ziemkiewicz notes. The ratios of one ion to another, however, tend not to change much over time, he says.
Produced water is highly saline. In samples tested at WVU, total dissolved solids (TDS) “rapidly increased to a maximum concentration of 100–150 g/L,” Ziemkiewicz says. TDS in produced water are primarily inorganic salts from the dissolution of fractured rocks. The combined concentration of radionuclides 226Ra and 228Ra also increases rapidly in the first year after a well is completed, reaching a maximum of 20,000 pCi/L, he adds. The EPA limit for the combined concentration of the two radium isotopes in drinking water is 5 pCi/L. The researchers found that the combined radium concentration correlated with the concentration of TDS. Over time, the concentrations of radium and TDS decrease, but that can take many years, Ziemkiewicz says.
Membrane treatment, such as reverse osmosis, or distillation is often necessary to remove the salts and radioactive substances before produced water can be used for agricultural purposes or discharged into surface waters. Such treatment processes are currently expensive. Researchers and natural gas companies are therefore seeking creative ways to use produced water.
In Pennsylvania and West Virginia, the reuse of produced water is common in the fracking industry. When drilling new wells, “we use roughly 30% produced water” mixed with fresh water from the Monongahela River, says Andy Travis, a spokesperson at Northeast Natural Energy, a natural gas producer in Morgantown, West Virginia. In Pennsylvania, it is common for natural gas companies to share produced water with one another.
Reuse is also growing in the Permian Basin in West Texas and New Mexico, “where disposal options have been or may become limited and disposal costs have been high or are increasing,” according to the GWPC.

The “reuse of produced water works great” as long as companies are establishing new wells at a high rate, Ziemkiewicz says. “If you are no longer completing many wells, you don’t have a market for produced water, and it starts accumulating,” he says. The drilling of new natural gas wells is slowing down because of low gas prices, and that trend is expected to continue in 2020, according to the Energy Information Administration.
WVU researchers are examining the feasibility of an alternative—adding untreated produced water to wastewater from thermoelectric power plants to help lower the costs of treating the power plant wastewater. Civil and environmental engineering professor Lian-Shin “Lance” Lin is leading the work.
Thermoelectric power plants, such as coal-fired and nuclear facilities, heat large amounts of fresh water to produce pressurized steam that drives a turbine to generate electric power. The steam loses pressure but remains warm, so it is mixed with cooling water and goes through a heat exchanger and cooling tower to dissipate the thermal energy.
A significant amount of water evaporates during the cooling process, which leaves behind water that contains higher concentrations of scale-forming substances, such as calcium and magnesium, Lin says. Over time, calcium carbonate will precipitate and foul the heat exchanger, he notes. When that happens, “you take some water out for treatment,” he says. The water that is removed for treatment is called blowdown water.
Treating blowdown water is expensive, Lin says. The process involves softening the water by adding chemicals to remove scale-forming substances, followed by either reverse osmosis or thermal desalination to remove the salts, which can be sold as a concentrated brine solution. But when produced water from the fracking industry is added to blowdown water, barium sulfate and calcium carbonate precipitate immediately, leaving behind water that is less costly to treat, Lin says. “Mixing the two gives you some degree of softening right away,” he says.
The mixture is still processed in a softening unit to remove multivalent cations, including radioactive substances from the produced water, but it requires fewer chemicals than blowdown water alone, Lin says.
Adding produced water, which is high in TDS, to blowdown water also increases the concentration of TDS in the blowdown water. That high-TDS blowdown water can be turned into a saturated brine solution, which can be sold for industrial and oil-field applications. Making that brine could use less energy than making the brine without produced water, Lin says. Alternatively, the high-TDS brine solution can be treated using electrolysis to generate chlorine and sodium hydroxide, which are two other useful products, he notes.
So far, the researchers have shown that produced water from the Morgantown Industrial Park, an MSEEL natural gas–producing site operated by Northeast Natural Energy, can reduce the costs of softening blowdown water from a local power plant in Morgantown. The next step is to show the energy savings in the thermal desalination step, Lin says. The team is also exploring whether it is more economical to truck blowdown water to the produced water or vice versa. It may also be possible to truck the mixed water to a treatment facility, he notes.
“The beauty of this,” Lin says, is that “if this works, you take away the produced-water problem altogether.”
As researchers investigate alternative ways to treat and use produced water, the fracking industry is urging the EPA to allow more produced water to be discharged to surface water and water treatment facilities. The Marcellus Shale Coalition, an industry group with members in the Marcellus and Utica Shale regions in Ohio, Pennsylvania, and West Virginia, wants the EPA to set effluent limit guidelines (ELGs), such as limits for TDS, for produced-water discharges.
“The water treatment industry has demonstrated that produced water can be treated to water quality concentrations that would be acceptable for discharge into surface waters,” the group says in comments submitted to the EPA in June. Concentrated brines can be separated from discharged water and “marketed to and reused in other industries,” the group says. “The Agency needs to set reasonable ELGs for the industry and allow science and economics to determine the best reuse or water treatment options for the natural gas industry to utilize.”
Produced water could also help alleviate water shortages that are common in some parts of the US. “Water stress is becoming a significant problem, especially in the arid west,” the GWPC says in comments submitted to the EPA. Earlier this year, the GWPC released a report evaluating current regulations and practices for managing produced water, as well as the research needed to turn produced water into a valuable resource.
“We are concerned about the overuse of fresh groundwater resources and wanted to explore how produced water might help fill that gap,” Mike Paque, GWPC executive director, said in June when the group’s report was released. The oil and gas industry handles about 55% of produced water as wastewater, according to the GWPC report.
Some academic researchers, however, are not convinced that current treatment options sufficiently remove radioactive substances from produced water. In particular, radium can accumulate in sediments and food sources, such as clams and mussels, Pennsylvania State University civil and environmental engineering professor Nathaniel Warner says in comments submitted to the EPA. “Prior to allowing an increased volume of produced water to be discharged, EPA should consider studying radium accumulation,” specifically downstream of oil and gas wastewater discharges, he says.
Advertisement
Environmental groups argue that the EPA should strengthen its rules on produced water, not weaken them. The agency’s draft study “indicates the need to increase protections, rather than changing regulations in order to facilitate more discharge,” a coalition of 33 clean water groups says in comments submitted to the EPA. The groups raise concerns about the unknown chemical composition of produced water and the potential for radioactive and other toxic substances. Reproductive and developmental toxicity data are lacking for the majority of the chemicals used by the fracking industry. Environmental groups also point to problems, such as agricultural land contaminated with salt, that have already occurred from discharges of produced water and unintentional spills.
The EPA is expected to finalize its produced-water study and decide whether to allow increased discharges of produced water later this year.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter