Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Water
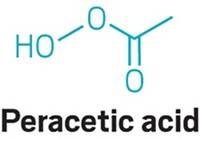
How peracetic acid is changing wastewater treatment
A new disinfection chemistry holds environmental and financial promise
by Craig A. Bettenhausen
April 19, 2020
| A version of this story appeared in
Volume 98, Issue 15

For decades, the city of Memphis, Tennessee, didn’t disinfect its wastewater. It stopped disinfecting in the 1980s because of concerns that residual chlorine would harm aquatic life and because of the cost of neutralizing the chlorine. It relied instead on settling, filtration, and microbial digestion for treatment. But in the first decade of this century, when its discharge permits were coming up for renewal, the city started working with environmental engineers on a new way to disinfect its discharge into the Mississippi River: peracetic acid.
In 2015, Memphis asked companies for bids to supply peracetic acid to its main wastewater plant. One of them, PeroxyChem, responded with a bigger pitch. The firm wanted to operate the city’s disinfection system and build a peracetic acid plant next door.
The city agreed. PeroxyChem built the plant and late last year started making peracetic acid on-site. The plant is large enough to supply both of Memphis’s wastewater plants as well as other peracetic acid markets, such as food safety. The deal also gives other Tennessee wastewater plants access to peracetic acid at lower costs than they could get elsewhere.
Peracetic acid at a glance
▸ 16,000 municipal wastewater plants in the US treat 45 billion L of sewage each day.
▸ 90% of peracetic acid’s disinfection activity happens in the first minute.
▸ Peracetic acid is a stronger oxidant than sodium hypochlorite and chlorine dioxide but weaker than ozone.
▸ In returnable containers, peracetic acid costs around $1.40–$1.80 per liter.
▸ UV light and metal contaminants strongly reduce peracetic acid stability.
▸ Most peracetic acid is sold in 15 or 22% concentrations and used at 1–2 ppm in wastewater.
Sources: Domenico Santoro, Kati Bell, Alberto Garibi, Jacquelyn Wilson, Lenntech, Tom Warmuth.
Today, chlorine dominates the US wastewater treatment market, disinfecting sewage at more than two-thirds of the country’s 16,000 plants. But the various chlorine-based chemicals used in water treatment create disinfection by-products that can harm the environment and human health. Residual chlorine flowing out of treatment plants can kill aquatic life. Tightening regulations on disinfection by-products and residual chlorine are driving up costs for wastewater plants using those workhorse chemistries.
For some plants, peracetic acid is the answer. It isn’t known to create harmful disinfection by-products. And because peracetic acid decomposes quickly into acetic acid, oxygen, and water, there is little need to remove or neutralize it before treated water enters waterways. As more plants adopt peracetic acid, its cost is decreasing. Once a niche product, peracetic acid may soon challenge the dominance of chlorine in large swaths of the US wastewater treatment market.
The global peracetic acid market was worth $650 million in 2017 and will grow to $1.3 billion by 2026, according to the consulting firm Trends Market Research. That estimate includes other uses of peracetic acid, such as poultry processing and industrial cleaning. Peracetic acid for wastewater treatment specifically is expected to grow by 8% per year over that same time frame.
Memphis will be a big part of that growth. In November 2018, the 356 million L per day Maynard C. Stiles Wastewater Treatment Facility started using peracetic acid supplied by PeroxyChem—the world’s largest such system. In February of this year, the big German chemical maker Evonik Industries purchased PeroxyChem for $640 million, largely on the strength of its peracetic acid business. Evonik expects Memphis’s other wastewater plant, which handles 264 million L per day, to start using peracetic acid in November.
“We have a long-term contract with the city of Memphis that enabled us to invest with a bit more peace of mind in this new production facility,” says Alberto Garibi, a PeroxyChem executive who now leads Evonik’s water and wastewater treatment business. “And it reduced operating costs for the city.”
Memphis’s wastewater stream is challenging, Garibi explains, because it contains a lot of industrial wastewater in addition to the municipal flow. The amount of chlorine required to meet the disinfection targets would be cost prohibitive, he says.
Many large wastewater plants looking to avoid chlorine choose disinfection with intense ultraviolet light. It’s effective and costs less than peracetic acid over time, though UV systems are expensive to install, explains Kati Bell, managing director of water strategy for the environmental engineering firm Brown and Caldwell, who worked on the Memphis project. But UV wasn’t a good fit for Memphis because the industrial waste component makes the water dark in color, and UV light can’t penetrate it well.
“Where we have these difficult effluents, that’s where peracetic acid seems to shake out,” Bell says. Right now, peracetic acid has less than 1% of the US wastewater market, but she expects it will eventually reach 10–15%.
A lot of the gain will be with smaller facilities, she says. One reason is that it’s relatively cheap for most small plants to convert from chlorine to peracetic acid. Berkeley Heights, New Jersey, installed permanent peracetic acid tanks and pumping equipment in February after 3 years of lab tests and pilot runs. BioSafe Systems is providing the township’s peracetic acid. The plant handles about 7.5 million L per day of wastewater.
Facility director Tom McAndrew says the conversion cost was $45,000, a huge savings over the $1 million quotes he got for UV. His employees, in consultation with an engineering firm, handled the construction themselves.
With the permanent setup, he says, the township should save a lot of labor and hassle. He also expects electricity and maintenance costs to go down because the facility has one pump, for peracetic acid, instead of one for chlorine and another for sodium bisulfite to neutralize the chlorine.
And during the facility’s time with peracetic acid delivered in skid-mounted totes, returnable containers that carry around 1,200 L, McAndrew says, it was saving 10–12% on chemical costs. Bulk deliveries to the new tank should save even more.

Another New Jersey town, Mount Holly, is working now on permanent equipment to replace the totes from PeroxyChem it currently uses. The 11 million L per day plant has been using peracetic acid for 3 years to serve about 15,000 households. Robert Maybury, executive director of the Mount Holly Municipal Utilities Authority, says the town has saved at least $35,000 per year since switching from chlorine.
And the price of peracetic acid is dropping as the market grows. “We’re even finding that some of our suppliers who used to supply us with sodium hypochlorite . . . they’re all starting to bring on peracetic acid,” Maybury says. “There’s a lot more competition.”
Other industries are also increasing their use of peracetic acid disinfection, which is helping increase supply and lower costs, says Jacquelyn Wilson, technical sales manager at Enviro Tech Chemical Services, a peracetic acid manufacturer. Her firm and competitor BioSafe Systems started out in markets such as food safety, industrial cleaners, agriculture-pathogen control, fracking-water disinfection, and algae control in lakes and ponds. “It’s still not quite at commodity pricing,” Wilson says, “but more and more, it’s widely used.”
Mount Holly’s main reason for switching was changes to the town’s discharge permit that forced it to drastically reduce bromodichloromethane and residual chlorine levels. Berkeley Heights made its move earlier, in anticipation of such changes, which New Jersey is implementing statewide.
Peracetic acid is also going into small plants that are maxed out because of population growth in the areas they serve, says Tom Warmuth, who covers lake, pond, and municipal markets for BioSafe Systems. Because peracetic acid works quickly and efficiently, they can get the same level of disinfection on a larger volume of water without increasing the plant size.
Peracetic acid isn’t perfect. Maybury says chlorine was better at controlling algae in Mount Holly’s plant, and now workers have to periodically drain and scrape some parts of the system. Many microbes eat acetic acid, which is a part of the equilibrium mixtures that peracetic acid comes in and is what it decomposes into as it does its work.
That phenomenon can make peracetic acid a bad fit for plants designed with a long disinfectant contact time. Wilson says 30–45 min is ideal. More than that, and you have to battle microbe regrowth. But, she says, engineers can often adapt peracetic acid to a treatment plant with a long contact chamber by moving the injection point.
Wilson enjoys challenges like that. “Everyone’s got something different,” she says. “That’s the really fun thing about what I do here. It’s not just a drop-in that’s going to solve all your problems. You have to know what your background water chemistry is” and tailor the system to the specific plant.
Peracetic acid works by oxidizing essential parts of microbes, often via reactive oxygen species such as ∙OH radical. Research into mechanisms is ongoing, but peracetic acid is known to disrupt sulfur bonds, so it’s great for breaking up proteins and peptides, says Ching-Hua Huang, a professor of environmental engineering at Georgia Institute of Technology. Peracetic acid seems to penetrate cells better than H2O2 and some other oxidants, which may be why it kills so quickly.
Some of Huang’s research is on wastewater systems that use UV simultaneously with peracetic acid, part of an approach called advanced oxidation (Environ. Sci. Technol. Lett. 2018, DOI: 10.1021/acs.estlett.8b00249).
UV disinfects by damaging the DNA and RNA of microbes and viruses. UV also spurs peracetic acid to produce additional reactive oxygen species. This boosts efficacy while allowing the oxidation of pollutants that peracetic acid can’t normally touch, such as pharmaceuticals. But more work is needed to understand the kinetics, reactivity, and selectivity in those systems, Huang says.
Several plants use peracetic acid ahead of UV irradiation chambers. “A lot of the wastewater treatment plants in the US that use UV struggle when you have wet weather,” because of an increase in solids in the incoming water, Bell says. “You start to see particle shielding. It’s like a little umbrella for microorganisms.”
By breaking up those solids and bleaching dark-colored chemicals, peracetic acid can make UV more effective. “It’s not all about replacing chlorine,” Garibi says. “It’s about seeing peracetic acid as another tool in your toolbox.”
One threat on the horizon for the peracetic acid market is viruses, a topic Wilson is asked about a lot in the current COVID-19 crisis. “I hate to break it to you,” she says, “but no facility currently tests for any virus,” including SARS-CoV-2, the one that causes COVID-19.
Enviro Tech’s peracetic acid products are effective against the new coronavirus, Wilson says, but the data the firm has are for hard contact surfaces, for which the World Health Organization includes peracetic acid among recommended SARS-CoV-2 killers.
A desire for virus control could bring competition from performic acid, a related peracid that companies including USP Technologies, a peroxygen specialist, are investigating for use in wastewater. Performic acid has a lot of the advantages over chlorine that peracetic acid has while being more effective against viruses.
Studies that USP conducted suggest that performic acid might also be cheaper to use, says Domenico Santoro, a senior manager for research and innovation at the firm. It currently has to be prepared on-site, he says, but that means “anyone who can supply formic acid and H2O2 could be a supplier.”
Bell anticipates guidelines on viruses from the US Environmental Protection Agency as early as 2022, which would trickle down into wastewater plant discharge permits a few years later.
Right now, acceptance of peracetic acid by regulators is state by state. North Carolina is allowing only bench-scale tests, whereas in New Jersey and Tennessee, some plants are able to get the go-ahead right away, Wilson says. Denver’s biggest plant has been in a full-scale trial of peracetic acid with Enviro Tech since early 2018, and city officials say they hope to make their switch permanent by the end of the year.
And though it took years for treatment plants like Memphis, Denver, and Berkeley Heights to get regulatory approval to adopt peracetic acid, they paved the way for plants looking at the switch now.
A year in, “Memphis is going great,” Garibi says. The peracetic acid facility is on line, and the disinfection system is meeting discharge standards. Evonik is fine-tuning its dose control automation, working out bugs in the system, and learning how to cope with sudden temporary flow increases during storms.
“One of the important barriers for us is that people in the municipal market are very data driven,” Garibi says. “At the beginning, there was not a lot of efficacy data available. We heard many times, ‘We don’t want to be serial number 1.’ But as more and more plants adopt it, we see the rate of change increasing.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter