Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
New membrane could reinvent crude oil refining
Rigid, porous material overcomes swelling and could slash energy used to separate crude oil into its parts
by Prachi Patel, special to C&EN
July 17, 2020

Crude oil is a complex mixture of tens of thousands of organic hydrocarbon molecules, Separating them requires energy-intensive thermal distillation processes that consume about 1% of global energy. Researchers now report the first polymer membrane capable of splitting crude oil into separate parts, a much more energy-efficient approach (Science 2020, DOI: 10.1126/science.aba9806).
While this is an early-stage demonstration, the possibility of membrane-based crude oil separation hints at “an opportunity to drastically reduce the energy intensity of the crude oil and chemical processing industry,” says Ryan P. Lively, a chemical engineer at the Georgia Institute of Technology.
Around 100 million barrels of crude oil are processed every day at oil refineries. As crude oil is pumped through enormous distillation towers and heated, lighter molecules with lower boiling points such as gasoline and diesel vaporize first and rise to the top. Meanwhile, heavier oils separate at the bottom. The heating, pumping, and condensing all require significant energy use.

“Membrane systems typically consume about 10 percent of the total energy of distillation columns,” Lively says. Membranes are used on a large scale to purify and desalinate water. And a relatively new class of microporous polymer membranes is on the cusp of being commercialized for splitting gas mixes, but they can’t handle crude oil. They absorb the small liquid organic molecules in the mixture and swell, which makes them unable to separate molecules of similar sizes and shapes.
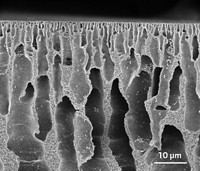
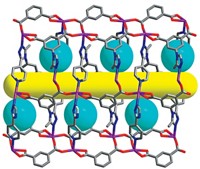
Lively, chemist M. G. Finn, and their colleagues made their membranes using spirobifluorene aryl diamine polymers. The building block monomers of the polymers are connected to each other via N-aryl bonds. “These connecting points are not oriented in a line, they’re kinked structures,” says Finn. This kinked structure provides what he terms “a happy medium of properties.” It makes the membrane highly porous and relatively rigid, just flexible enough to let small liquid molecules through while resisting swelling.
The team coated a thin film of the polymer on a large roll of a porous plastic support. The membrane could separate light crude oil into two parts, one containing lighter molecular weight compounds and the other containing heavier molecules. “We imagine that ultimately you won’t have one magic membrane,” Lively says. “You’d have a portfolio of membranes that would separate different fractions.”
There are several challenges ahead in developing a membrane for crude oil separation on a large scale, Lively says. Foremost is increasing the flux, the amount of liquid that can pass through the membrane per unit area. The team also plans to study fouling and aging of the membranes over long-term use, though the membrane showed no fouling after two months of continuous testing with light crude, Lively says.
Improving flux will be crucial to decrease the number and cost of membrane modules that are needed for separation, says Joan Brennecke, a chemical engineer at the University of Texas at Austin. Heavier crude oils will likely cause fouling issues, though, and the use of membranes might be limited to light crude oils, she says. Still, solving the membrane swelling problem is a major step forward. “This is an important breakthrough,” she says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter