HOW CHEMISTRY THRILLS IN
AMUSEMENT
PARKS
Roller coasters, holograms, cotton candy, and haunted houses exhilarate, thanks to advanced materials and chemicals.
Illustration by Alexander Wells
Roller coasters are not for everyone, but for those who love them, they can become a lifelong passion.
Colin Coon is not only a roller-coaster superfan; he also designs them. A design engineer, Coon works at Rocky Mountain Construction, a manufacturer specializing in wooden and hybrid roller coasters. The company, in fact, built the world’s first multi-inverting wooden coaster in 2013 and the world's tallest wooden roller coaster in 2014.
Coon spends his days dreaming up bigger, faster, and crazier coasters, but chemistry is at the heart of everything he does—just as it is for everyone who makes amusement parks the magical places they are.
Not your father's roller coaster
At RMC, Coon gets to indulge in both fantasy and nostalgia. He imagines new roller coasters and brings them to life while also turning aging wooden coasters into state-of-the-art attractions. “We don't just copy the old layout of the original coaster,” he says. “We add higher and steeper drops and multiple inversions to provide a new, thrilling experience.” Coon and his colleagues do this by replacing the original wooden track with a steel-track system, allowing for higher speeds and lower maintenance.
If it seems like coasters are bigger and more exciting than before, that's because they are. Advanced materials science lets engineers like Coon build bigger thrills. Steel components, for example, are galvanized by submerging the bare steel into molten zinc. “The iron in the steel reacts with the molten zinc to form an alloy coating, thus giving it incredibly high corrosion protection,” Coon says. This protection is important to keep the ride smooth, reliable, and thrilling for many years.
Building bigger, safer hybrids of wood-and-steel structures or all-wood roller coasters requires the right lumber. Coon explains that hard-to-break southern yellow pine is the wood of choice. The wood is placed in a vacuum chamber, where all the air is sucked out and moisture is removed. Next, to prevent decay, fungal growth, and insect infestation, alkaline copper quaternary and chromated copper arsenate are pumped in under pressure to force them into the wood.
When designing roller coasters, Coon explains, builders make it impossible for the train to jump the track. There are three sets of wheels on the train and three running surfaces on the track.
The correct application of lubricating grease is a balancing act that plays a vital role in controlling the speed of the ride. Perhaps surprisingly, too little grease speeds up the train, while too much slows it down, even causing it to grind to a halt mid-ride. Coon typically uses a lithium-based grease which has the consistency of peanut butter at room temperature but changes to a liquid state as the wheels heat up.
“Adding grease means there is more grease to absorb the generated heat from the bearings, so the grease stays thicker in consistency,” he explains.
Thicker grease makes for a slower ride. But, by contrast, if there is less grease to absorb the heat, the grease itself heats up and thins out.
“The thing I find most exciting and thrilling about designing new roller coasters is seeing which limit we are going to push next,” Coon says. “Just this year we built a 205-foot-tall [62.5-meter] record-breaking monster of a roller coaster and two highly innovative next-gen Raptor roller coasters.”
Like their dinosaur namesakes, these Raptors are nimble and fast, designed to reach heights of over 61 meters and speeds of up to about 121 km/hour. The Raptors have a single rail, whereas most coasters have two. The track is just over 30.5 cm wide, Coon explains, and each passenger is seated in his or her own car, in single file. People's legs are positioned on either side of the track, meaning they straddle it as they ride.
It's a 3-d world
The roller coasters Coon builds will always be an amusement-park favorite, but new technologies, such as virtual reality and holograms, allow designers to blend real and fantasy worlds in novel ways. Some modern rides already feature holographic characters, and researchers are employing cutting-edge chemistry to push such light technology even further.
Currently, “fabricated on a flat panel, a hologram appears to be 3-D to the viewer,” says Alexander R. Lippert, a chemist at Southern Methodist University. “If the viewer looks at the back of the holographic plate, they see the back of the plate, not the back of the 3-D image.”
Looking forward, though, Lippert and his team have been shaping light in space to generate volumetric three-dimensional displays, which enable a viewer to see a 3-D image or animation from any angle. Each voxel (or 3-D pixel) in the display is a point of fluorescence emission created by using a fluorescent photoswitch, such as N-phenyl spirolactam rhodamine B, to control the light emission.
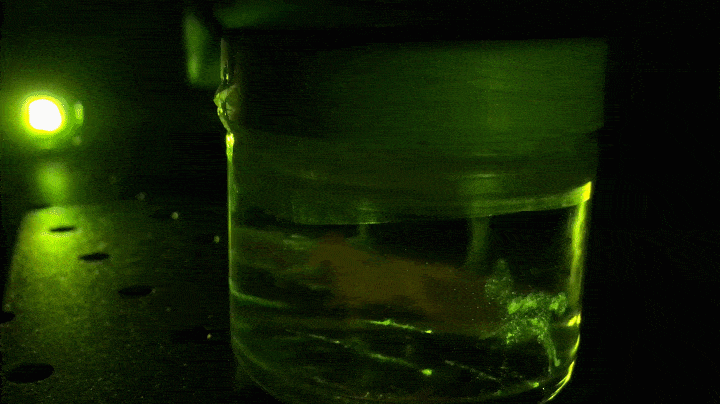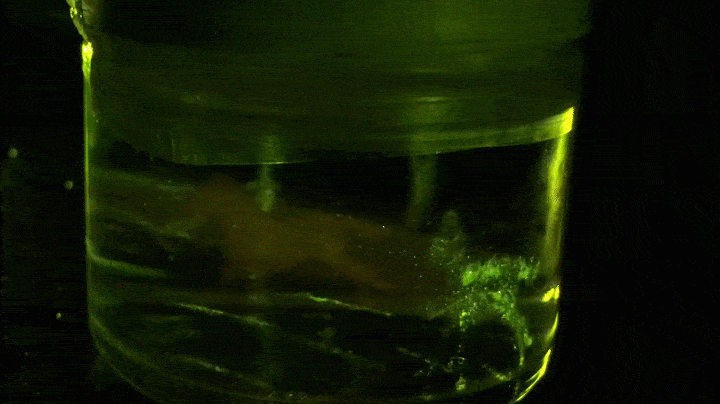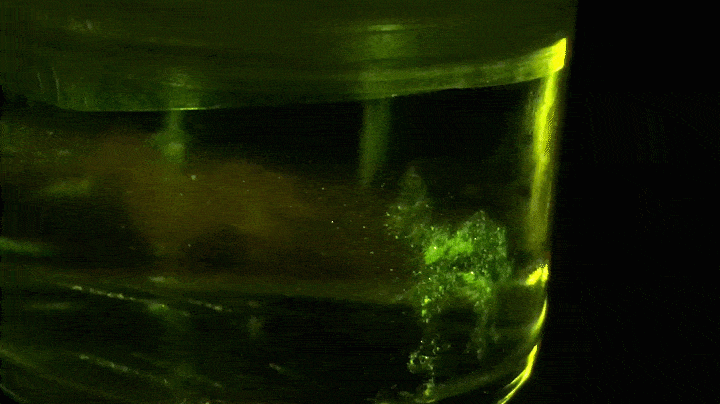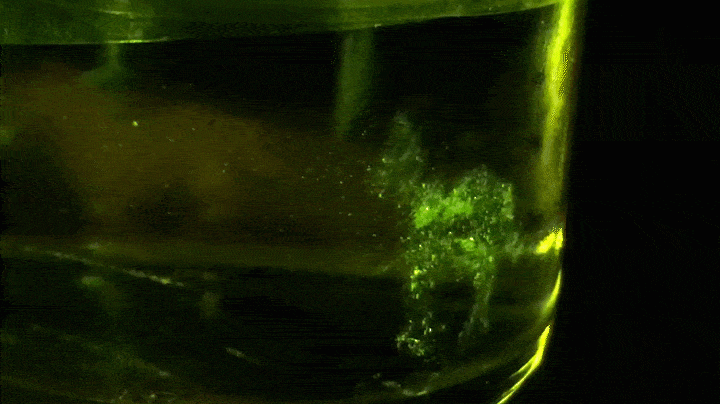
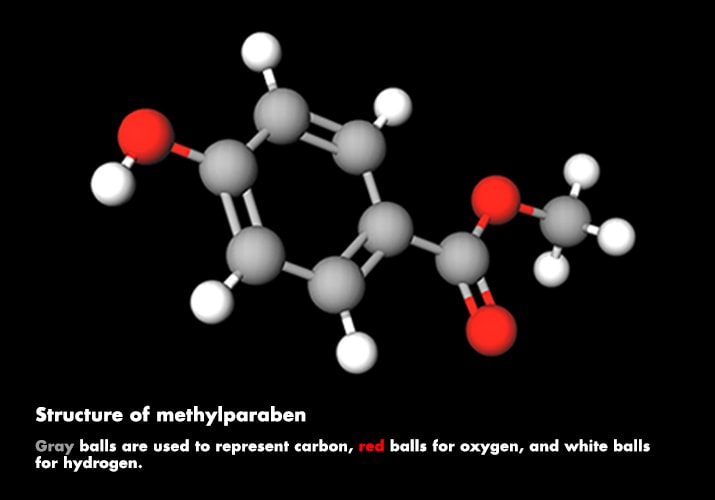
In the dark, the photoswitch is a closed conformation and colorless. It becomes fluorescent only when ultraviolet and visible light hit it simultaneously. Excitation by UV light initiates a photochemical reaction that opens the photoswitch, allowing it to absorb the visible light and fluoresce. “If the photoswitch diffuses off the path of the ultraviolet beam of light, it quickly changes its conformation back to the colorless, closed form,” Lippert says. In short, it can be turned on and off.
To make 3-D images, the team created a setup known as a 3-D Light PAD. The setup relies on the photoactivatable dye inside a 50-mm3 quartz glass cube with two projectors and a camera arranged around the cube. Lippert and his colleagues dissolve the fluorescent photoswitch into a solution inside the cube, then project UV and visible light into the cube. A spatial pattern of fluorescent emission (that is, the 3-D image) appears at the intersection of the two light beams.
Lippert believes this technology could have many applications, including biomedical imaging and 3-D videoconferencing. However, he was inspired by the idea of making something fun and entertaining. On one of Lippert's favorite amusement park rides, passengers wear 3-D glasses as they travel through stereoscopic scenes and shoot at moving targets to score points. Lippert loves the experience, but he recognized “how the stereoscopic 3-D scene broke down a bit at the corners of your field of view. I would get a little nauseous after wearing the glasses for a while.” Lippert thinks the 3-D display technology his team is developing could overcome these problems.
Triggering fear without danger
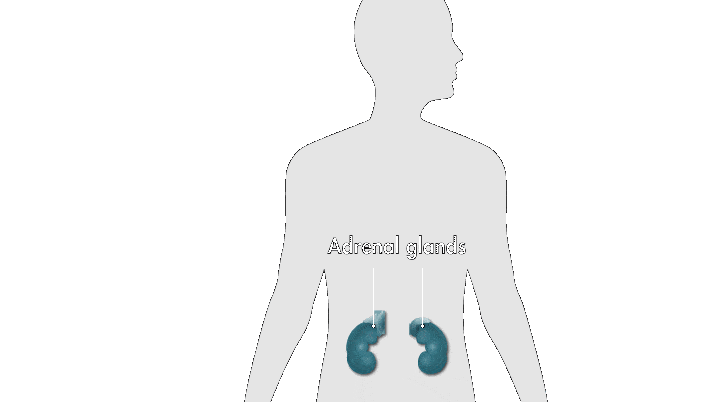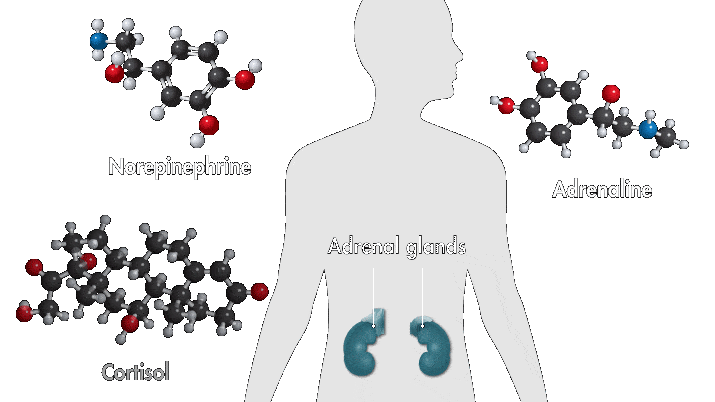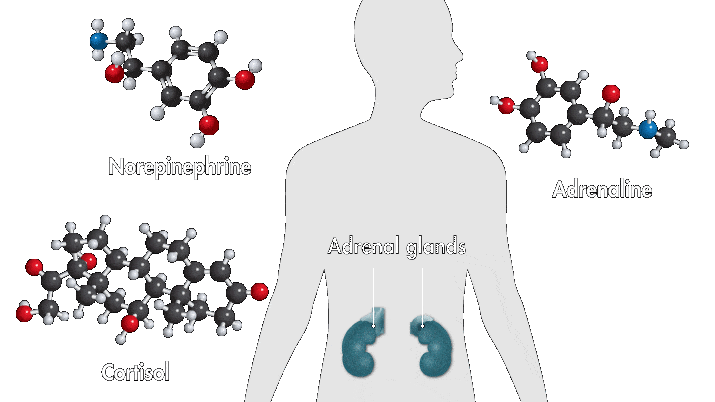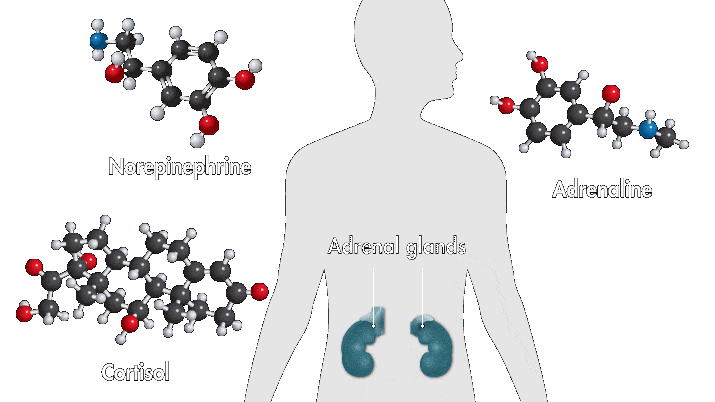
One reason amusement parks thrill is because they toy with your sense of danger. The high-speed twists, turns, and loop the loops of a roller-coaster ride produce a thrilling buzz. Key ingredients of that buzz are stress hormones, including adrenaline and cortisol. In response to danger or threat, the brain releases neurotransmitters that activate nerves in the body that trigger the secretion of these hormones.
Epinephrine—a type of aromatic amine that circulates in the blood—performs a number of functions when you encounter danger. When it enters the bloodstream and reaches the heart, it binds to cardiac β1 receptors, which cause the heart to beat faster. Meanwhile, cortisol, a glucocorticoid, pumps glucose to muscles and narrows the arteries, also raising heartbeat. These chemical reactions, alongside other physiological changes, such as faster breathing, are among the sensations associated with fear.
These intense physical sensations can be pleasurable, even addictive. That's where exhilaration and thrill come in. While the chemistry is similar, what really makes fear different from exhilaration and thrill is our cognitive interpretation, explains Joseph E. LeDoux, neuroscientist at New York University and author of “The Emotional Brain.”
Fear is the cognitive awareness that you are, or believe you are, in harm's way, he explains. It is a construction based on sensory input, memory, and self-representations, as well as brain arousal and feedback from the body. When these factors coalesce into awareness, the result is conscious fear. As LeDoux explains, “We get the intense exhilaration—which we label as fear—on rides but without the actual expectation of harm.” That is why that thrilling adrenaline rush feels so good. On a ride, you feel the fear, but you know that you're not in danger.
From cotton candy to artificial organs
No trip to an amusement park is complete without a taste of cotton candy. This cloud of baby-pink or light-blue fibers is formed when disaccharide and monosaccharide sugars are melted in a cotton candy machine. Forces holding the sucrose molecules together break during melting and produce a liquid that can be spun into ultrathin threads of sugar. This process has surprising applications in chemistry.
A team from Vanderbilt University has shown that cotton candy machines can be used to spin fibers into networks of capillary-like structures, which could support the production of artificial organs. “If you want to build artificial tissue that's thicker than a couple of human hairs, you need to have a sort of internal plumbing that provides nutrients and gas exchange for all the cells within. Otherwise, they're going to die,” explains Leon M. Bellan, a mechanical and biomedical engineer who heads the team.
Building this internal plumbing requires channels in the gel scaffold in which the cells grow. To make the capillary-like structures, the team builds a sacrificial structure of fibers using a thermoresponsive polymer called poly(N-isopropylacrylamide), which dissolves when cooled but remains insoluble when warm gelatin is poured over it.
Bellan's previous experience working on nanofibers inspired him to use a cotton candy machine to spin these fibers. Nanofibers are about one one-thousandth the thickness of a human hair, but Bellan sought something thicker for this particular project. Cotton candy threads, he realized, are the exact thickness needed.
The team spins its thermoresponsive fibers in the cotton candy machine and then pours warm liquid gelatin mixed with cells over them. The gelatin is also mixed with an enzyme called microbial transglutaminase, which causes it to set. The result is a matrix of solidified gel with thermoresponsive fibers and cells embedded inside. Once it cools to room temperature, the thermoresponsive fibers dissolve, leaving behind perfectly capillary-sized channels, which provide the “internal plumbing” required to support the cells.
Fake blood; real chemistry
As an amusement park aficionado, Coon loves the thrill of being spooked on a ghost train as much as he loves riding roller coasters. “Knoebels Grove in Elysburg, Pa., has a homemade haunted house ride that is, hands down, one of the best in the world,” he says.
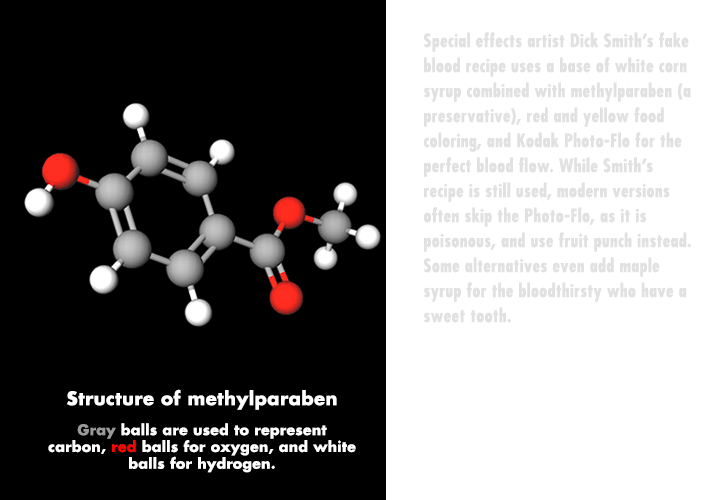
Haunted houses and ghost trains draw on special effects, such as prosthetics and fake blood, to create monsters, spooks, and zombies. Makeup and special-effects artist Dick Smith, who worked on classic movies such as “The Exorcist” and “The Godfather,” invented one of the classic fake blood recipes. Smith's method combines a base of white corn syrup with methylparaben (a preservative), red and yellow food coloring, and Kodak Photo-Flo for the perfect blood flow. His recipe, and variations of it, is still used today.
To make ghoulish masks and rotting zombie heads, special-effects designers often make molds from gypsum cement, which is stronger and more durable than plaster. The cement retains detail and can be used many times over. Elastic masks are made by brushing liquid latex into the mold and letting it set. Solid prosthetics, such as for hands and feet, can be made by pouring foam latex (composed of a latex base and foaming and gelling agents) into a mold, then heating it in an oven, causing it to harden.
Designers also use silicone rubber, a colorless elastomer to which pigments can be added, to create realistic flesh tones. Platinum-cured silicone is especially popular, as it has high tear resistance and low shrinkage properties—useful for detail and accurate reproduction. Some silicone products are also safe to apply directly to an actor’s skin, making them handy for building gashes and wounds.
Coming full loop the loop
Amusement parks are a wonderful nexus of playfulness and high-end chemistry that allow everyone to let loose, except, perhaps, parents with motion sickness. Fortunately for roller-coaster devotees, Coon suffers no such malady. He took to the rides at an early age. For his eighth birthday, Coon rode the giant wooden coaster, Gwazi, at Busch Gardens. “I was terrified at first, but once the ride got going, I absolutely loved it,” he recalls.
Thanks to Gwazi, Coon developed a lifelong passion. His enthusiasm for his work is infectious. “Every day is different here,” he says. “I love it.”
Precipitating the conversation:
A Perspective From Diane Closser (LeftTop) And Chad Warriner (RightBottom), Technical Service Engineers At The Chemours Company


It's frustrating when a machine you depend on breaks down. And you really want dependability when the machine is an open car and you're in it, careening down a 400-foot drop at speeds approaching 130 miles an hour.
Roller coasters and other amusement park rides get more extreme with each passing season, but they also continue to post outstanding safety records. This is thanks in part to the use of the latest technologies and products with remarkable properties. The International Association of Amusement Parks and Attractions estimates that theme parks are around 7 million times safer than going for a drive.
Bigger, better thrills
Park rides, like other innovative, high-demand technologies, need improved greases like Krytox™ to increase uptime, reliability, and safety, which keeps the merry-go-rounds spinning, the floats parading, and the roller coasters zooming. Krytox™ could improve the lubrication of rotating equipment that contains bearings, gearboxes, and electric motors. Because Krytox™ can withstand extreme temperatures, attraction designers can create new rides that continue to use the advances of materials science and engineering while pushing the limits of human endurance. Krytox™ doesn't break down, volatilize, or ignite, thereby enabling designers to invent bigger, better thrills.
Enabling the everyday
Science can be found all around amusement parks, from the food chemistry of cotton candy to the biochemistry of an endorphin rush to the environmental chemistry of a water park. Similarly, Krytox™ can be found all over the everyday world. Its high heat tolerance and stability make it ideal for everything from the lubrication of the moving parts in ovens to aerospace applications. It helps bicycle chains run smoothly and keeps your car rattle- and squeak-free.
Krytox™ has been around for over 50 years, but we're still finding new applications and formulations for it. Just as the hunger for thrills pushes park rides to new heights, the demand for efficiency and performance drives people to push their machines to greater and greater extremes. And as the hills they set out to conquer get bigger, we will be right there—where Krytox™ can make all the difference.
READ HOW CHEMOURS HELPS MAKE AMUSEMENT PARKS COLORFUL AND SAFER:
October 1: The Chemistry of Sports
November 26: The Chemistry of Travel
December 17: The Chemistry of Dining
HOW CHEMISTRY ROCKS MUSIC FESTIVALS
PACKING THE RIGHT MOLECULES FOR THE GREAT OUTDOORS
ABOUT SPONSORED CONTENT
Sponsored content is not written by and does not necessarily reflect the views of C&EN's editorial staff. It is authored by writers approved by the C&EN BrandLab and held to C&EN's editorial standards, with the intent of providing valuable information to C&EN readers. This sponsored content feature has been produced with funding support from The Chemours Company.