Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Zeolite membrane finds holey purpose in methanol synthesis
Porous sodium aluminosilicate separates water from gases in high-temperature reaction
by Mark Peplow
February 6, 2020
| A version of this story appeared in
Volume 98, Issue 6
A porous crystalline membrane that selectively pulls water molecules out of a gaseous reaction mixture could improve the efficiency of industrial processes such as methanol synthesis (Science 2020, DOI: 10.1126/science.aaz6053).
Chemical separations, including distillation and evaporation, account for up to 15% of global energy demand, and developing more efficient separation methods, such as those involving porous membranes, could make a big dent in greenhouse gas emissions. But separation membranes often need to be exquisitely selective: water molecules have a diameter of 2.6 Å, for example, while hydrogen gas is 2.9 Å wide, so distinguishing between the two is a big challenge.
Polymer membranes are already used to tease apart mixtures of chemicals, but they tend to be less selective than porous inorganic crystals called zeolites. A zeolite called sodium aluminosilicate (NaA) is used in industry as a molecular sieve to remove water from organic solvents such as ethanol, but defects in its structure can allow the wrong molecules to sneak through, especially at high temperatures and pressures. “Nobody has used NaA membranes for water-gas separation under high-temperature and high-pressure reaction conditions,” says Miao Yu at Rensselaer Polytechnic Institute.
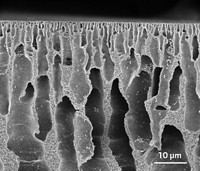
Yu’s team has now developed an alternative approach for making robust, high-quality NaA membranes. They took 30-cm-long ceramic straws riddled with pores roughly 400 nm across and coated them with NaA seed crystals that were 50–200 nm wide. A blast of heat caused the nanocrystals to chemically bond to the ceramic support and fill its pores with zeolite. This effectively created a cylindrical NaA membrane that was 4 µm thick and remarkably free of defects, which the researchers attribute to the size and quality of the seed crystals.

The membrane’s gas permeability is hundreds or thousands of times lower than previous NaA membranes, yet its water permeability is hundreds or thousands of times higher than its gas permeability. That means water can travel much more easily than gases through the 4.2 Å-wide channels in the zeolite. Once through the channels, water eventually condenses on the outside of the membrane.
The team’s calculations suggest that two effects work together to enhance the membrane’s selectivity for water. Certain sodium ions in the crystals partially block the nanochannels, which reduces the effective size of the pore. Meanwhile, other sodium ions along the channel help to pull polar molecules like water through more readily than carbon dioxide or H2.
The researchers tested the performance of the membrane in a reaction that combines CO2 and H2 to produce methanol and water. The NaA zeolite itself does not catalyze this reaction, so the researchers loaded three of their membrane-coated straws with a few grams of a commercially available copper-zinc-alumina catalyst. Then they ran a mixture of CO2 and H2 through the straws at 250 ºC and 35 bar. Removing water helps to shift the reaction equilibrium toward the products, improving the yield of methanol. The reactor produced about three times as much methanol as an equivalent assembly that lacked the water-separation membrane.
The membrane offers a couple of other advantages. Without the membrane’s separation abilities, the methanol produced in this reaction is very wet, and would have to be purified by distillation. But the membrane ensured that the methanol dripping from the end of the reactor was 95% pure. Removing water from the reaction stream also protected the catalyst from degradation, and the reactor could run for more than 100 hours with only a slight decrease in methanol yield.
“It’s a very powerful example that illustrates how porous crystals grown as membranes can enable industrially relevant chemical separations at remarkably high performance,” says Moises A. Carreon at Colorado School of Mines, who develops porous materials for separation, gas storage, and catalysis.
Further tests need to show that the membranes can perform well under industrial conditions, which may involve higher pressures and gas streams that are contaminated with traces of hydrocarbons, carbon monoxide, or sulfides. “But if these can be shown, this membrane certainly has the potential to be deployed for industrial applications,” Carreon says.
Yu has founded a start-up company called E2H2NANO to scale up the process and is making membrane straws that are 50–80 cm long to assess their performance.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter