Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biomaterials
Spider silk proteins form hydrogels at body temperature
The previously unobserved phenomenon could lead to tissue engineering and drug delivery applications
by Payal Dhar, special to C&EN
September 7, 2022

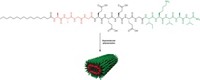
Spider silk proteins known as spidroins can form hydrogels at body temperature, according to a new study. The study’s authors think that the gels could be tailored for a variety of biomedical applications, such as tissue engineering and drug delivery (Nat. Commun. 2022, DOI: 10.1038/s41467-022-32093-7).
Hydrogels are 3D polymer networks that can hold large amounts of water. Spider silk is biocompatible and doesn’t tend to trigger immune reactions in people, making the materials safe for biomedical purposes, says study first author Tina Arndt, a researcher at Karolinska Institute.
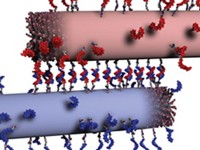
Arndt and her colleagues discovered that spidroins could form hydrogels by chance. The scientists had sent a sample of spidroins made by bacteria to collaborators, but the package was delayed in transit. When it finally arrived, the protein sample had gelled unexpectedly. On analyzing the different parts of the protein, they found that its gel-forming ability was due to the spidroin’s N-terminal (NT) domain, the amino acids at the start of the protein chain.
Spidroins are normally stored as a soluble liquid in the spider’s silk gland. When the spider starts to spin its web, the proteins undergo structural changes that cause them to solidify. Specifically, the proteins’ C-terminal (CT) domain, which is at the other end of the protein chain from the NT, unfolds, converting from a helical form to an amyloid-like one.
Researchers had previously assumed the NT domain always remained stable and soluble. Arndt and colleagues, however, found that at high enough concentrations and at normal body temperature, the NT does undergo structural changes while forming a hydrogel.
In lab experiments, the researchers created fusion proteins by combining the NT domains with other proteins, including an enzyme. They found the fusion proteins retained their biological functions.
R. Helen Zha, who studies bioinspired and biomimetic materials at the Rensselaer Polytechnic Institute, calls the discovery of this new spider silk ability fascinating. The hydrogels reported in this study are incredibly versatile in that they can easily incorporate a variety of bioactive proteins, she says.
Arndt and colleagues are working to develop an injectable protein solution that turns into a hydrogel inside the body for drug delivery and tissue regeneration applications.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter