Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biomaterials
Supramolecular polymers treat spinal cord injury in mice
Motion of monomers plays key role in targeting receptors
by Celia Henry Arnaud
November 12, 2021

There are no currently available ways to reverse spinal cord damage. Researchers are working to fix that by trying to develop methods to regenerate neurons following spinal cord injury. A team has now moved closer to that goal with supramolecular polymer threads that successfully treat spinal cord injury in mice. Properly tuning the mobility of the individual molecules within the threads, the researchers say, proved to be a key characteristic leading to the successful healing of nerve damage in mice.
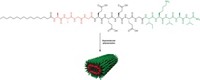
Samuel I. Stupp of Northwestern University and coworkers made the supramolecular fibers—tube-shaped assemblies of molecules with one end pointing to the center of the tube and the other pointing out. (Science 2021, DOI: 10.1126/science.abh3602). Each molecular building block has multiple segments. At the outward-facing end is a peptide that activates a targeted receptor associated with nerve growth. An alkyl tail at the other end drives assembly of the supramolecular structure into a squashed tubular shape. In between are segments that make the structure water soluble and others that control mobility of the individual molecules within the supramolecular structure.

To design the supramolecular material to aid in regenerating nerve fibers in damaged spinal cords, the researchers tested structures with varying amounts of molecular mobility and incorporated different amounts of two signaling peptides. One signal promotes the regeneration of nerve fibers. The other signal promotes cell proliferation and blood vessel formation. The ideal combination carried 90% of the former and 10% of the latter, the researchers found.
Incorporating two signals “is not a trivial thing to do,” Stupp says. “It’s not like you mix two molecules and that’s it.” That’s where the sequences that control mobility came in. The amount that the individual molecules could migrate to other locations within the assembly correlated with the amount of signaling that the researchers observed. The molecules carrying the signaling peptides both had some degree of mobility, but the researchers chose mobility-determining sequences that weakened interactions between the two types of building blocks. That allowed the ones with the cell-proliferation signals to cluster together.
Stupp thinks the collective motion is important because it allows the cell-signaling peptides to better interact with their target receptors. “These peptides have to touch the receptors in a specific spot in order to activate the receptor and then trigger the signaling pathway,” he says. The receptors are moving around in the cell membrane, Stupp says. “If the therapy is moving also, the probability that they will encounter each other in the right way to activate the signal is much higher.”
To treat spinal cord injury in mice, the researchers injected an aqueous solution of the supramolecular polymer into a mouse’s spinal cord. When they come in contact with the spinal cord, the nanofibers form a hydrogel that mimics the spinal cord’s extracellular matrix. Mice who received supramolecular polymers with the right combination of motion-control sequences for the two signaling peptides had much more nerve fiber regrowth, blood vessel formation, and functional recovery than ones who received polymers that restricted signal mobility.
“The development of dynamic and bioactive scaffolds is still in its infancy,” Charlotte A. E. Hauser, an expert on biomedical applications of supramolecular materials at King Abdullah University of Science and Technology, writes in an email. “The observations made by the authors in this study could open the door for new avenues and materials to be explored by researchers for tissue engineering research.”
The current formulation is intended to treat acute spinal cord injury. “We are getting ready to approach the [US Food and Drug Administration] in early 2022 with our preclinical work to obtain permission for human trials,” Stupp writes in an email. Stupp and coworkers also plan to develop other treatments for chronic spinal cord injury and brain injuries.
CORRECTION
This article was updated on Nov. 18, 2021, to correct the presentation of Charlotte Hauser's name. Her name is Charlotte A. E. Hauser, not Charlotte A. Hauser.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter