Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Inorganic Chemistry
Abundant ingredients make CO₂ reduction more practical
Carbene catalysts plus alkali metals are key to the new process
by Leigh Krietsch Boerner
July 23, 2020
| A version of this story appeared in
Volume 98, Issue 29
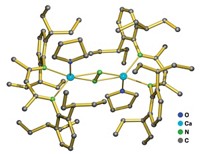
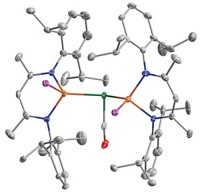
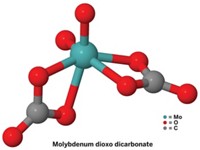
To make it practical to transform CO2 into something more useful, researchers are trying to develop catalytic reactions that use cheap and abundant ingredients. Robert J. Gilliard Jr. and coworkers at the University of Virginia and Latrobe University have uncovered a key step in this process, unlocking electrons from CO2’s strong π-bonds by binding them to cyclic (alkyl)(amino)carbenes (ChemRxiv, 2020, DOI: 10.26434/chemrxiv.12665336.v1). They then used three earth-abundant alkali metals to reduce the stubborn CO2 bond. ChemRxiv is a preprint server and the paper has not been peer reviewed.
“CO2 by itself does not react with things like alkali metals,” Gilliard says. Once the carbene forms a zwitterionic adduct with the CO2, the researchers add solid lithium, sodium, or potassium to form alkali metal clusters. Depending on how much metal they add, the CO2 is either singly or doubly reduced. The process works at atmospheric temperatures and pressures, sidestepping the need for hard-to-handle or expensive reagents or catalysts. The resulting clusters’ valence orbitals aren’t completely filled and they have interesting reactivities. Using earth-abundant metals for this reaction makes it more applicable for future CO2 reduction technologies, Gilliard says.
The approach is unique and beautiful in its simplicity, says Robert Mulvey, an inorganic chemist at the University of Strathclyde. The chemistry can lead to high-value chemicals and materials such as methanol and dimethyl ether. It’s also a remarkable example of how broadly chemists can apply these types of carbenes, according to Guy Bertrand, an organometallic chemist at the University of California San Diego. “These results should encourage chemists to develop catalysts based on carbon rather than on transition metals,” he says.
Correction
This story was updated on July 24, 2020, to correct the type of carbene the researchers used and the type of adduct formed in the study. The carbene was a cyclic (alkyl)(amino)carbene, not an imidazolium 2-carboxylate carbene. The adduct was a zwitterionic adduct, not a zwitterion adduct.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter