Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Inorganic Chemistry
Actinide orbitals drive C–C coupling
Reaction creates actinide 2-metallabiphenylenes
by Leigh Krietsch Boerner
March 7, 2020
| A version of this story appeared in
Volume 98, Issue 9
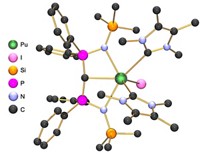
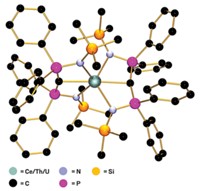
Researchers have found a new kind of aromatic molecule whose unusual electronic properties may open up new realms of reactivity. Jaqueline Kiplinger at Los Alamos National Laboratory and colleagues there and at the University of Vermont and the University of Minnesota Twin Cities have made the first known actinide 2-metallabiphenylene compounds through metal-mediated reductive coupling (Nature 2020, DOI: 10.1038/s41586-020-2004-7). The actinides’ 5f and 6d orbitals set off a C–C coupling reaction to form cyclobutadiene rings, sandwiched between a benzene ring and the metallacyclopentadiene; the molecule (shown) obeys Hückel’s rule. Spectroscopy and calculations suggested that in both uranium and thorium systems, the cyclobutadiene is antiaromatic, and the benzene is aromatic. However, the team found that the U 5f orbitals covalently bond with the metallabiphenylene, whereas the Th does not because it has no f electrons. “The 5f and 6d orbitals on the actinide metals are enabling chemistry that the rest of the periodic table hasn’t been able to achieve,” Kiplinger says. This highlights the differences between the transition metals and the actinides, says Trevor Hayton, an inorganic chemist at the University of California, Santa Barbara, “and suggests that many more exciting reactions are waiting to be discovered.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter