Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Inorganic Chemistry
Chelating agent selectively grabs uranium from oceans
Inspired by nature, new adsorbent soaks up uranium from seawater, leaving interfering ions behind
by Mitch Jacoby
February 21, 2019
| A version of this story appeared in
Volume 97, Issue 8

The world’s oceans contain some 4 billion metric tons of dissolved uranium. That’s roughly 1,000 times as much as all known terrestrial sources combined, and enough to fuel the global nuclear power industry for centuries. But the oceans are so vast, and uranium’s concentration in seawater is so low—roughly 3 ppb—that extracting it remains a formidable challenge. That task may have just become easier thanks to a new adsorbent material based on a bioinspired chelating agent (Nat. Commun. 2019 DOI: 10.1038/s41467-019-08758-1).
Researchers have been looking for ways to extract uranium from seawater for more than 50 years. In the 1980s, surveys pointed to amidoxime-type chelating agents, which have a knack for latching onto uranyl ions, the aqueous form of uranium.
Nearly 20 years ago, the Japan Atomic Energy Agency (JAEA) confirmed that amidoxime-functionalized polymers could soak up uranium reliably even under harsh marine conditions. But that type of adsorbent has not been implemented on a large scale because it has a higher affinity for vanadium than uranium. Separating the two ions raises production costs.
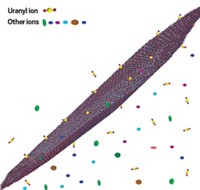
Alexander S. Ivanov of Oak Ridge National Laboratory, together with colleagues there and at Lawrence Berkeley National Laboratory and other institutions, may have come up with a solution. Using computational methods, the team identified a highly selective triazine chelator known as H2BHT that resembles iron-sequestering compounds found in bacteria and fungi. Starting with low-cost reagents, the team prepared fibers containing polyethylene and polyacrylic acid, functionalized them with H2BHT (shown), and analyzed their performance as adsorbents.
H2BHT exhibits little attraction for vanadium but has roughly the same affinity for uranyl ions as amidoxime-based adsorbents do. And in contrast to amidoxime adsorbents—which are tough to recycle because of the acid treatment needed to further purify uranium they gather—the new adsorbent can be regenerated with mild carbonate solution and reused.
JAEA’s Masashi Kaneko offers high praise for the study. H2BHT’s high selectivity and uranium uptake capacity, coupled with molecular insights from the team’s analyses, may lead to improved methods for recovering uranium from seawater, he says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter