Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Inorganic Chemistry
Elusive phosphorus compound synthesized
Long-sought phosphatetrahedrane could lead to new organophosphorus compounds
by Celia Henry Arnaud
April 2, 2020
| A version of this story appeared in
Volume 98, Issue 13

Tetrahedranes are highly strained hydrocarbons with tetrahedral cores. Such molecules could serve as high-energy materials and building blocks, but they are difficult to make and isolate.
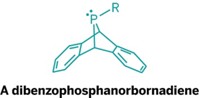
Christopher C. Cummins and coworkers at the Massachusetts Institute of Technology thought that compounds in which phosphorus replaced one of the carbons would be less strained and thus easier to capture. Cummins and his team have now made such a phosphatetrahedrane (Sci. Adv. 2020, DOI: 10.1126/sciadv.aaz3168). Their compound has tert-butyl substituents at the three carbon vertices, a strategy that has been previously used to stabilize the hydrocarbon analog.
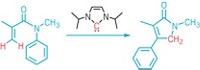
To make the phosphatetrahedrane, the researchers synthesized a cyclopropenyl precursor with a phosphorus atom attached to anthracene. They hoped that the anthracene would act as a leaving group and cause the precursor to close up into the desired phosphatetrahedrane. But when they eliminated the anthracene through protonation and the addition of fluoride, they got a cyclopropenyl-fluorophosphine, a phosphorus-containing precursor to a carbenoid analog. Treating that compound with lithium tetramethylpiperidide generated the target compound, which was stable enough that the researchers could obtain a crystal structure.
J. Chris Slootweg, a phosphorus chemist at the University of Amsterdam, says the s-orbital character of the phosphorus atom’s lone pair of electrons suggests that the compound will be an interesting ligand for coordination chemistry and catalysis.
“The isolation of a phosphatetrahedrane sets a milestone in organophosphorus chemistry,” says Robert Wolf, a chemist at the University of Regensburg whose group synthesized a related diphosphatetrahedrane. “This work is of interest to both organic and inorganic chemistry and will likely inspire significant subsequent chemistry. As the field develops further, new applications of such phosphatetrahedranes as reagents or reactive intermediates may also arise.”
Cummins’s group plans to cut out some steps in the synthesis by skipping the anthracene leaving group, since it wasn’t involved in the final step. “All we need to be able to do is to generate that phosphinidenoid. It doesn’t matter how we make it,” Cummins says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter