Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Metal-Organic Frameworks
MOFs strike tumors in mice with two-fisted blows
Drug-loaded compound combines radiotherapy and immunotherapy tumor treatment
by Mitch Jacoby
April 5, 2018
| A version of this story appeared in
Volume 96, Issue 15

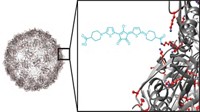
Like a boxer delivering a potent one-two punch to an opponent, chemists have used nanosized platelets of a metal-organic framework (MOF) compound to strike a double blow against tumor cells (Nat. Biomed. Eng. 2018, DOI: 10.1038/s41551-018-0203-4). The study may lead to improved treatments for some types of cancers and helps extend MOF usage beyond its most common areas of application—gas storage and separation.
MOFs are porous crystalline materials composed of metal clusters and organic linkers. Because chemists can readily tailor MOF structures and compositions—researchers have made more than 20,000 MOFs to date—it’s possible to customize the materials’ properties to suit a given application.
That flexibility, coupled with a focus on nanomedicine, motivated University of Chicago chemist Wenbin Lin and coworkers to examine MOFs for anticancer applications. The team found that MOFs built from hafnium clusters and porphyrin ligands can fight tumors in two ways—through radiotherapy and immunotherapy.
On the radiotherapy side, the team found that irradiating the MOFs with X-rays generates two types of tumor-fighting reactive oxygen species. The hafnium clusters efficiently absorb X-ray photons and produce hydroxyl radicals from water. The radiation also drives the porphyrin units to excite oxygen molecules from the triplet state (the ground electronic state) to the energetic singlet state.
The team also found that the pores and channels of the same MOFs could be used effectively to store and release a drug known as an IDO inhibitor. These drugs provide immunotherapeutic benefit by activating white blood cells known as T cells to attack tumor tissue.
The team injected the drug-loaded MOFs into tumors in mouse models of breast and colorectal cancer and exposed the tumors to X-rays. The MOFs generated reactive oxygen species effectively enough to eradicate tumors at much lower X-ray dosages than used in standard radiotherapy treatments. Reducing radiation exposure can minimize X-ray damage to healthy tissue.
As an added benefit,the MOF treatment also destroyed nearby tumors that were not exposed to X-rays through release of the IDO inhibitor, which triggered T cell activation.
RiMO Therapeutics, a start-up company founded by Lin, has recently begun Phase I clinical trials of RiMO-301, a related nanosized MOF developed in his lab, for treating tumors. For now, the company is not releasing detailed information about that MOF’s structure and composition.
“This impressive study effectively exploits the chemical diversity of metal-organic frameworks by combining the X-ray absorbing characteristics of the metal with an enzyme inhibitor small-molecule payload,” says University of Liverpool MOF specialist Matthew J. Rosseinsky. “The MOF chemistry directly enables this sophisticated approach,” he adds.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter