Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Metal-Organic Frameworks
Wendy Lee Queen
Materials monarch is combining MOFs with polymers to create composites that clean up air and water
by Bethany Halford
August 14, 2020
| A version of this story appeared in
Volume 98, Issue 31

Credit: Samuel Betrisey (Queen); J. Am. Chem. Soc. (gold);
Wide open spaces have always been a draw to explorers, and molecular prospector Wendy Lee Queen is no exception. Queen, a materials chemist at the Swiss Federal Institute of Technology, Lausanne (EPFL), has staked her claim on the vast frontier offered by metal-organic frameworks (MOFs). By filling this landscape with polymers, Queen makes composites that can capture all manner of molecules with the goal of cleaning up air and water and extracting valuable metals like gold from waste streams.
It can be tough to access all the chemical functionality in long polymer strands, which can stick together like cooked, unstirred spaghetti. MOFs, on the other hand, are large jungle gym–like structures with massive open spaces that excel at soaking up large volumes of chemicals. Queen’s group combines the two by building polymers inside MOF frameworks. This separates the polymer strands, so it’s possible to access the polymer backbone’s chemical functionality.
Advertisement
“As chemists, we can fine-tune these materials to selectively grab certain small molecules from gases or from air,” Queen says. “Or we can design those materials to extract certain things from water, for example highly toxic species.” Her team has made polymer-MOF composites that can pull carbon dioxide and water from air.
They’ve also made composites that remove mercury and lead from water. “Our materials can selectively grab those highly toxic species and leave behind less toxic ones in solution,” she says, even if other ions like sodium and potassium are around in much higher concentrations.
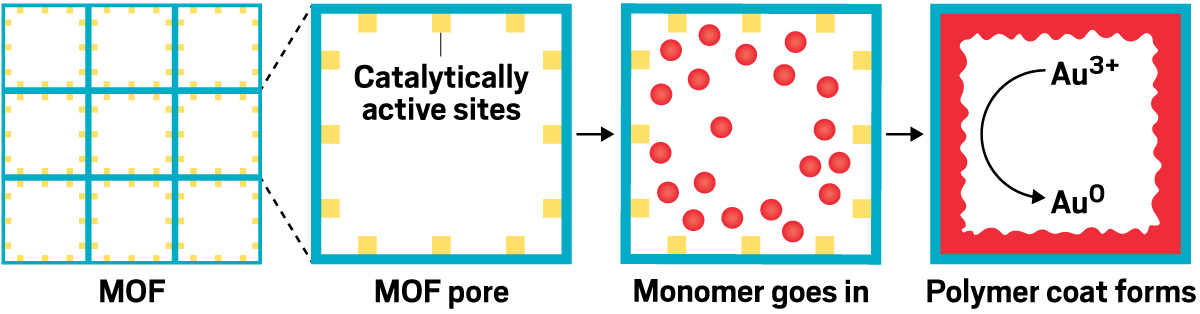
With one composite, Queen and coworkers were able to grab gold ions from a solution of electronics waste and reduce them to make shiny 23.9-karat gold nuggets. A gram of the composite can extract nearly a gram of gold. Now Queen is looking for ways to make the polymer-MOF composites into beads that could be used in filter cartridges.
“Wendy Queen is a brilliantly creative scientist, who has quickly established a world-leading research program in materials chemistry,” says Jeffrey R. Long, a chemist at the University of California, Berkeley, who was one of Queen’s postdoctoral mentors. “Her work stands poised to revolutionize the production and recycling of critical metallic elements.”
Queen wasn’t always interested in materials, or even chemistry for that matter. A softball scholarship helped her pay for college, where, after two years undecided on a major, she chose to study chemistry because she liked the challenge.
Her decision to go to graduate school was spurred by a professor who insisted she apply after she told him she had no interest in an advanced degree. “I was the first person to get a college degree in my family, and I guess because I really didn’t know anything about graduate school, I just assumed that I wasn’t cut out for it,” Queen says.
After earning her PhD from Clemson University, she did postdoctoral work at the National Institute of Standards and Technology, during which she spent some time working with Long in California. Following a 3-year stint at Lawrence Berkeley National Laboratory, she started her position at EPFL in 2015.
Even though she lives in Switzerland, Queen still makes time for America’s pastime by playing baseball in a Swiss league.
Vitals
Current affiliation: Swiss Federal Institute of Technology, Lausanne (EPFL)
Age: 39
PhD alma mater: Clemson University
Hometown: Anderson, South Carolina
If I weren’t a chemist, I’d be: A baseball coach. “When I was a little girl, I wanted to be a professional baseball player.”
If I were an element, I’d be: Carbon. “It takes very different forms: you have black coal, which is kind of rough and ugly, yet it can blossom into a beautiful polished gem. I imagine myself and my career as a diamond in the rough to some regard. It has taken a lot of hard work and self growth to get to the point that I was ready to take on the endeavor that I am today.”
Wide open spaces have always been a draw to explorers, and molecular prospector Wendy Lee Queen is no exception. Queen, a materials chemist at the Swiss Federal Institute of Technology, Lausanne (EPFL), has staked her claim on the vast frontier offered by metal-organic frameworks (MOFs). By filling this landscape with polymers, Queen makes composites that can capture all manner of molecules with the goal of cleaning up air and water and extracting valuable metals like gold from waste streams.
Vitals
▸ Current affiliation: Swiss Federal Institute of Technology, Lausanne (EPFL)
▸ Age: 39
▸ PhD alma mater: Clemson University
▸ Hometown: Anderson, South Carolina
▸ If I weren’t a chemist, I’d be: A baseball coach. “When I was a little girl, I wanted to be a professional baseball player.”
▸ If I were an element, I’d be: Carbon. “It takes very different forms: you have black coal, which is kind of rough and ugly, yet it can blossom into a beautiful polished gem. I imagine myself and my career as a diamond in the rough to some regard. It has taken a lot of hard work and self growth to get to the point that I was ready to take on the endeavor that I am today.”
It can be tough to access all the chemical functionality in long polymer strands, which can stick together like cooked, unstirred spaghetti. MOFs, on the other hand, are large jungle gym–like structures with massive open spaces that excel at soaking up large volumes of chemicals. Queen’s group combines the two by building polymers inside MOF frameworks. This separates the polymer strands, so it’s possible to access the polymer backbone’s chemical functionality.
“As chemists, we can fine-tune these materials to selectively grab certain small molecules from gases or from air,” Queen says. “Or we can design those materials to extract certain things from water, for example highly toxic species.” Her team has made polymer-MOF composites that can pull carbon dioxide and water from air.
They’ve also made composites that remove mercury and lead from water. “Our materials can selectively grab those highly toxic species and leave behind less toxic ones in solution,” she says, even if other ions like sodium and potassium are around in much higher concentrations.
With one composite, Queen and coworkers were able to grab gold ions from a solution of electronics waste and reduce them to make shiny 23.9-karat gold nuggets. A gram of the composite can extract nearly a gram of gold. Now Queen is looking for ways to make the polymer-MOF composites into beads that could be used in filter cartridges.
“Wendy Queen is a brilliantly creative scientist, who has quickly established a world-leading research program in materials chemistry,” says Jeffrey R. Long, a chemist at the University of California, Berkeley, who was one of Queen’s postdoctoral mentors. “Her work stands poised to revolutionize the production and recycling of critical metallic elements.”
Queen wasn’t always interested in materials, or even chemistry for that matter. A softball scholarship helped her pay for college, where, after two years undecided on a major, she chose to study chemistry because she liked the challenge.
Her decision to go to graduate school was spurred by a professor who insisted she apply after she told him she had no interest in an advanced degree. “I was the first person to get a college degree in my family, and I guess because I really didn’t know anything about graduate school, I just assumed that I wasn’t cut out for it,” Queen says.
After earning her PhD from Clemson University, she did postdoctoral work at the National Institute of Standards and Technology, during which she spent some time working with Long in California. Following a 3-year stint at Lawrence Berkeley National Laboratory, she started her position at EPFL in 2015.
Even though she lives in Switzerland, Queen still makes time for America’s pastime by playing baseball in a Swiss league.





















Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter