Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Nanomaterials
Chemists synthesize Saturn-shaped molecule
Flat, macrocyclic host captures fullerene guest with CH–π interactions
by Bethany Halford
June 17, 2018
| A version of this story appeared in
Volume 96, Issue 25

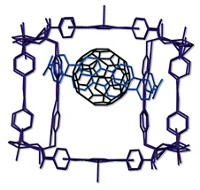
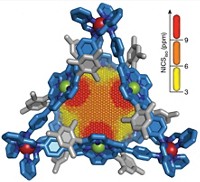
Saturn and its extraordinary rings have captivated scientists since they first started peering through telescopes. Chemists have now created a molecular homage to this heavenly body by building a so-called nanoSaturn out of just carbon and hydrogen (Angew. Chem. Int. Ed. 2018, DOI: 10.1002/anie.201804430).
A team led by Tokyo Institute of Technology’s Shinji Toyota synthesized the supramolecular complex by trapping a C60 within a large, hydrocarbon ring composed of substituted anthracene units. Scientists previously captured fullerenes with belt-type hydrocarbons or disk-like thiophene-based macrocycles, but this is the first example of a nanoSaturn with a disk-type hydrocarbon ring. To make the molecular assembly, the chemists simply combine the hydrocarbon ring system with C60, and weak CH–π interactions hold the system together. The researchers determined the nanoSaturn’s structure using X-ray crystallography (shown). Toyota notes the macrocyclic compounds might be useful for extracting or sieving fullerenes, but that wasn’t his main motivation. “What is most exciting for me,” he says, “is that this beautiful and harmonized shape can arouse the curiosity of any observer as planet Saturn does.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter