Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Nanomaterials
Photoactive enzyme can tame radicals
Reaction makes chiral carbonyls in high yields and selectivity
by Leigh Krietsch Boerner
June 21, 2020
| A version of this story appeared in
Volume 98, Issue 24

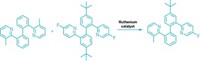
To control unruly radicals, sometimes mother knows best. Tapping into nature, Huimin Zhao and coworkers at the University of Illinois at Urbana-Champaign and Xiamen University in China have created a new enzymatic reaction that uses light to stereoselectively couple two carbon compounds (Nature 2020, DOI: 10.1038/s41586-020-2406-6). The reaction creates gamma chiral carbonyls, with ketones or esters, in 47 to 99% yields and with an enantiomeric selectivity of 93 to 99% (example shown). These types of terminal alkanes are difficult to make but found in many bioactive compounds, Zhao says. He and his team identified a class of enzymes called ene-reductases that can catalyze their target reaction. After optimizing the reaction, the researchers found that the known enzyme, old yellow enzyme 1, gave the highest yields of the product, with 96% enantiomeric selectivity, which surprised Zhao. “In an intramolecular coupling reaction, it’s hard to control the selectivity,” he says. Here, the enzyme provides a reaction vessel, forming only one enantiomer. The group proposes that the reaction has a novel, radical mechanism. The enzyme holds the reactants close to each other and stabilizes the radicals. The reaction is new to nature, Zhao says. Photoactive enzymes work under mild conditions and can access reactions elusive to chemical catalysts. This ene-reductase is so far the only catalyst that can do this selective reaction. Zhao thinks there may be more opportunities with similar chemistry. “There are many other flavin enzymes out there that are photoactive as well,” he says.
CORRECTION
This story was updated on June 25, 2020, to correct the journal name. The study was published in Nature, not Science.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter