Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Nanomaterials
Using designer proteins to make quantum dots
A synthetic protein catalyzes a chemical reaction that forms high-quality cadmium sulfide nanocrystals
by Prachi Patel, special to C&EN
December 28, 2022

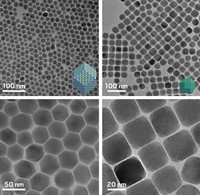
In a unique marriage of synthetic biology and chemistry, researchers have used custom-made proteins to drive chemical reactions that produce cadmium sulfide quantum dots (Proc. Natl. Acad. Sci. 2022, DOI: 10.1073/pnas.2204050119). Quantum dots are semiconductor nanocrystals that shine in a range of bright, pure colors depending on their size.
The cheapest way to make quantum dots today requires high temperatures and organic solvents. The new protein-catalyzed process works in aqueous solution at room temperature and produces high-quality quantum dots 2–5 nm in size.
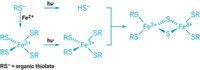
Chemists Michael Hecht, Gregory Scholes, and Sarangan Chari of Princeton University and their colleagues started with a designer protein made previously in their lab (Protein Sci. 2016, DOI: 10.1002/pro.2871). They discovered recently that this protein can catalyze the removal of sulfur from the amino acid cysteine to form hydrogen sulfide.
The team added in cadmium chloride and found that it reacts with the H2S to form CdS quantum dots. The reaction proceeds slowly, growing nanocrystals in a controlled manner, creating uniformly sized dots with optical properties matching that of conventionally produced ones. Longer reaction time led to larger nanocrystals.
This method to make quantum dots could open up new uses for the nanomaterial as biosensors and also lays the foundation for designing “made-from-scratch” proteins that perform chemical functions, Hecht says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter