Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Polymers
Pathogen-fighting polymer
Copolymer loaded with sulfonic acid groups kills bacteria and viruses in minutes
by Bethany Halford
August 11, 2019
| A version of this story appeared in
Volume 97, Issue 32

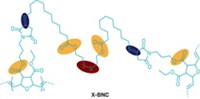
There’s a new weapon in the battle against bacteria and viruses—a polymer that kills pathogens on contact. The material could be used for biomedical applications, smart textiles, and food packaging. A team led by North Carolina State University’s Richard J. Spontak and Reza A. Ghiladi studied the pathogen-fighting abilities of the material—a commercially available copolymer in which two hydrophobic regions (shown in blue) bookend a hydrophilic region loaded with sulfonic acid groups (shown in red). The sulfonic acid groups decrease the pH at the polymer surface, and microbes coming in contact with the material experience a sudden drop in pH. This kills the microbes within just five minutes by destroying their membranes, damaging their enzymes, and denaturing their proteins. In tests, the polymer was effective against six types of bacteria, including a “superbug,” methicillin-resistant Staphylococcus aureus, which is a dangerous bacterium found on surfaces and equipment in hospitals and other medical facilities. The material also eradicated vesicular stomatitis virus, human adenovirus-5, and influenza A virus (Mater. Horiz. 2019, DOI: 10.1039/c9mh00726a). Although the polymer loses its potency over time, it can be revived by simply spraying it with an acidic solution like vinegar.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter