Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Drug Development
FDA drug approvals hit all-time high
The 59 new molecular entities approved include small molecules, biologics, and new modalities
by Lisa M. Jarvis
January 2, 2019
Last year was a busy one for the US Food and Drug Administration. Drug approvals hit an all-time high, with 59 new molecular entities securing the agency’s permission to head to market.
Small molecules continue to account for the lion’s share of new drugs, comprising 64% of therapies approved in 2018. Antibodies represented 20% of approvals. But with 15% of new drugs falling outside of those two main modalities, the list also reflects the increasingly diverse approaches researchers are using to take down diseases—particularly rare, genetic diseases.
Indeed, one of the highlights of the year was the approval of the first drug, Alnylam Pharmaceuticals’ Onpattro, that acts by RNA interference. Onpattro uses short, double-stranded nucleotides to intercept mRNA before it can deliver the instructions for assembly of an unwanted protein.
It was a scientific achievement, but the commercial viability of the product—and, more generally, RNAi technology—remains to be seen. Just two months after Onpattro made it onto the market, Akcea Therapeutics and Ionis Pharmaceuticals won approval for an antisense oligonucleotide for the same rare disease, hereditary transthyretin-mediated amyloidosis, addressed by Onpattro. And Pfizer has long worked on a small molecule for the disease.
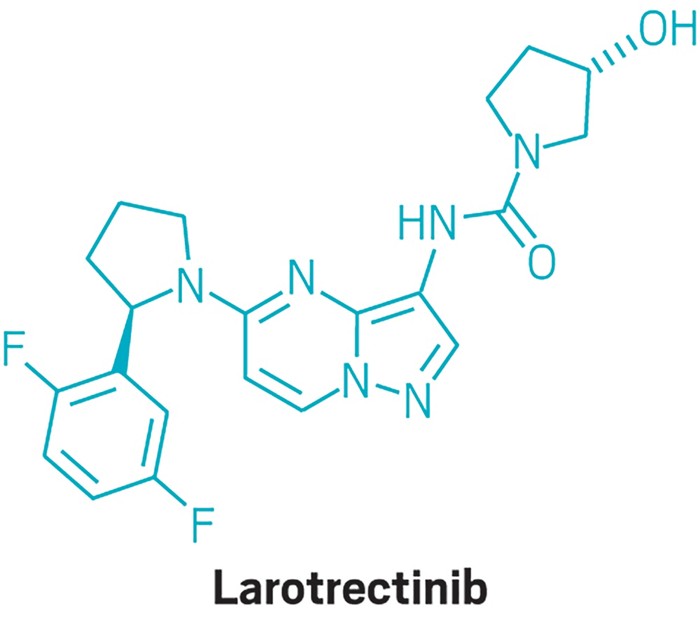
Another notable new drug was Loxo Oncology and Bayer’s Vitrakvi, which became the second cancer therapy to be approved that treats people based on their genetic profile, rather than location of their tumor. Overall, the FDA gave its nod to 16 new cancer therapies, an increase from the 12 cancer drugs approved in 2017. Of the oncology drugs approved, seven treat some form of blood cancer.
Beyond new modalities and cancer, last year’s class brought treatments for long-underserved diseases. Among the advances: a new class of drug for migraine prevention, the first new pill for endometriosis in over a decade, and the first marijuana-extracted treatment, Epidiolex, for a rare form of epilepsy.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter